Digging through the annals of chemistry, trimethylamine didn’t hide out for long. Its discovery in the 19th century happened as researchers poked and prodded through decomposition products from organic matter like fish and plants. This wasn’t just idle curiosity—there was a purpose to figuring out why old fish stank to high heaven. Those early days saw scientists isolating an ammonia-like substance with a stubborn, lingering odor, connecting it to organic breakdown. Over time, researchers started synthesizing it intentionally. By the 20th century, trimethylamine found firm footing in chemical industries, cropping up in practical applications, not just in theory work. Its journey from the mystery meat in nature to a tool on factory floors mirrors chemistry’s habit of turning oddities into everyday utilities.
Trimethylamine lands on the shelf as a colorless, volatile liquid, impossible to miss thanks to its strong, pungent smell—think rotting fish, or overripe seafood in a forgotten lunchbox. Chemical producers supply it in various concentrations, typically as aqueous solutions or liquified gas, packed in steel drums or cylinders. Along the supply chain, everybody knows to mind the risk—improper sealing or storage makes itself apparent real quick. On the business end, it shows up in everything from chemical synthesis packages to niche specialty formulations, with consistent sales to pharma, agri-chem, and coatings sectors.
Anyone handling trimethylamine notices its firepower. The boiling point hovers just above room temperature, at about 2.9°C under atmospheric pressure, which cranks up the volatility. Solubility in water runs high—meaning spills disappear into drains, just as manufacturers worry. Solvents like ethanol, ether, and chloroform absorb it eagerly. The vapor stings the nose and eyes, and a handful of lab incidents show that even diluted concentrations clear a room fast. The molecular structure (C3H9N) balances three methyl groups stuck to one nitrogen atom, lending it reactivity for methylation reactions. Its density sits at around 0.667 g/mL, and flammability sets off alarms in storage rules, especially at room temperature.
Chemicals don’t travel far without paperwork. Suppliers tag trimethylamine with UN number 1083, classifying it as a flammable, toxic substance. Safety Data Sheets always call out the need for proper ventilation and spill response. Labeling includes batch numbers, purity, concentration for solutions, and hazard pictograms. In the EU and US, transport labels follow strict GHS rules, with distinct wording for acute toxicity and environmental risk. Inspection teams often check containers for corrosion or leakage; any lapse shows up fast with this material.
Old methods leaned on heating fish brine or organic sludge, but those days are gone. Most modern trimethylamine flows out of chemical plants that react ammonia with methanol over an alumina catalyst at elevated temperatures around 350°C. This method balances atom economy with cost. Plant operators fine-tune ratios and reaction conditions to swing between mono-, di-, and trimethyl products, separating out trimethylamine by distillation. Byproducts get recycled or sold into lower-margin streams. The setup isn’t especially tricky, yet the equipment needs to stay tight and venting lined up for environmental and safety control.
Trimethylamine often plays the role of a nucleophile in alkylation reactions, taking in alkyl halides to build quaternary ammonium salts. This makes it a staple in surfactant and disinfectant synthesis. It reacts strongly with acids to form salts like trimethylamine hydrochloride, used in lab and industry. In organic chemistry, it acts as a base for neutralizing acids and pulling off esterifications or aminolysis steps. Chemists don’t just use it raw; modifications spawn all sorts of specialty compounds, from corrosion inhibitors to unique catalysts. Reactivity remains high, so handlers keep neutralizers and spill kits close.
This compound goes by several aliases. Chemical catalogs list names like TMA, N,N-dimethylmethanamine, +Trimethylamine anhydrous, and Methyltrimethylamine. Industrial grades might show up simply as “TMA gas” or “Trimethylamine solution” depending on composition. Each supplier sticks to nomenclature rules but never drops hazard identifications—nobody laughs off the odor or risk.
Anyone who has worked with TMA remembers the first time the smell hit them—the kind of memory that sticks. Exposure limits remain tight: OSHA’s permissible exposure set at 10 ppm as an 8-hour time-weighted average. Personnel require proper PPE—nitrile gloves, splash goggles, and in lab-scale mishaps, full-face respirators. Storage involves gastight lines, well-ventilated flameproof cabinets, and regular leak detection patrols. Fire marshals point out that TMA vapors travel and ignite readily, so grounding and bonding of all equipment holds importance. First-aid training for exposure or inhalation stays part of every SOP in organizations using the stuff.
Walking across sectors, trimethylamine pops up in places both obvious and surprising. Clearly, it earns its keep in the manufacture of choline chloride—an essential feed additive in the poultry industry. It’s pressed into duty making quaternary ammonium compounds, which slip into disinfectants, antistatic agents, and water treatment chemicals. Pharmaceutical chemists use it to build intermediates for drugs like antihistamines and local anesthetics. Downstream, even explosives and rocket propellant manufacturing pull in this compound for key synthesis steps. In gas-scrubbing operations, TMA helps strip acidic gases from process streams. Its reputation for a strong odor gets pressed into use in leak detection, where any whiff signals a breach. In the lab, its basicity wins favor for specialized synthesis steps.
Though trimethylamine has a long track record, new applications and improved methods spark frequent study. Process engineers focus on making the synthesis cleaner and safer, taking on catalyst breakthroughs or more efficient methanol recovery. Researchers look at greener sources—biofermentation from plant feedstocks—to address sustainability targets. Analytical chemists explore detection and capture strategies, aiming for sensors sensitive enough to monitor traces before numbers reach health limits. In product design, specialists tinker with derivatives for custom properties, filling new needs in rapidly evolving fields like biotechnology or polymer chemistry. Safety research links up with toxicologists to better understand exposure pathways and refine emergency handling guides.
Trimethylamine doesn’t pull punches on health. Acute exposure sends employees scurrying—respiratory irritation, itchy eyes, and sometimes headaches sound familiar to anyone caught downwind. Chronic studies have probed for broader health impacts, with some animal tests spotlighting potential for organ effects. Emerging research investigates the role of trimethylamine as a metabolite in the human gut, especially its conversion to trimethylamine N-oxide (TMAO) and links with cardiovascular risk. Community and occupational health teams keep the compound high on their air-monitoring checklists, and incident logs in manufacturing cite the need for solid containment. TMA gets flagged in environmental standards, since aquatic life takes a hit even at low-level spills.
Looking ahead, trimethylamine finds itself caught in a balancing act. On one side, its value in food supplements, coatings, and clean-tech products keeps the orders rolling in. On the other, tighter environmental and health scrutiny pushes chemical firms to dial in better containment and to cut fugitive releases in plants. Bio-based production methods have grabbed attention, with startups and traditional players both exploring microbial fermentation routes—reducing dependency on petrochemical feedstocks. Analytical innovation may impact how companies monitor background concentrations, both for regulatory compliance and worker safety. Fresh synthetic chemistry, especially in precision pharma or smart materials, could open up new uses, if risks stay under tight control. No matter which path leads, trimethylamine will continue stirring debate among policy makers, scientists, and industry hands alike.
Walk into a chemical plant or step outside a fishery and you’ll notice a sharp, almost fishy smell hanging in the air. That’s trimethylamine. Plenty of people recognize it without ever learning its name. This small molecule packs a big punch, and its uses stretch farther than folks might think. It’s not just something to avoid on a hot day at the docks; it plays a role in things most people touch every day.
Factories rely on trimethylamine to put together ingredients that end up in everyday products. It acts as a building block for other chemicals. Companies need it to make choline, which helps people and animals keep their brains and nervous systems working right. You’ll spot choline in animal feed—especially for chickens and pigs—since their growth depends on it. Walk through a feed mill and the unique scent of trimethylamine sometimes hangs in the background.
Open a bottle of household disinfectant and there's a chance trimethylamine stood somewhere in the production line. It helps make quaternary ammonium compounds—“quats” for short. These are the things that kill germs on kitchen counters and hospital surfaces. Without trimethylamine’s role in the supply chain, manufacturers run into a problem keeping bacteria and viruses in check.
The first time I read a safety sheet about trimethylamine was as a lab assistant, and the strong warning about its odor and volatility stood out. People can sense its presence with only trace amounts in the air. This is part of what makes it tricky to handle: a small spill won’t go unnoticed for long. For workers, it’s more than an inconvenience. Exposure can bring on watery eyes, headaches, or worse in confined spaces.
Beyond disinfectants, trimethylamine connects to other big industries. Fertilizer makers use it to help crop yields, sending nutrients deep into farming soil. Pesticide production also leans on it in certain formulations to keep insects from wiping out food supplies. Skipping trimethylamine isn’t an option for businesses trying to supply global agriculture.
Trucks hauling trimethylamine cross highways, sometimes close to towns. Accidents and leaks pose real risks, since the gas proves both toxic and highly flammable. Even beyond the threat of direct exposure, trimethylamine coming off decaying meat and fish upsets residents and workers, driving complaints. In the body, breakdown into smaller molecules can get tangled up in chronic diseases for people who can't process it fully. Medical researchers keep looking at ways to control or block these processes, since gut bacteria also turn foods like eggs and meat into small amounts of this chemical.
The future calls for smarter management and tighter rules. Chemical plants need better monitoring equipment that picks up leaks before they spread. Training workers to handle trimethylamine correctly isn’t optional. Emergency crews deserve regular drills and clear steps in case something goes wrong during storage or transit. Research might one day lead to substitutes for some uses, helping industries reduce risks and lower the amount released into the environment. Tackling trimethylamine’s challenges won’t be simple, but the pressure comes from all sides—workers, communities, and consumers who want both safety and clean products.
Trimethylamine floats around with a reputation for its fishy odor long before folks even talk about health risks. The smell hits hard—think of the scent near a fish market or certain cleaning supplies—and gets attention right away. People who have worked in fertilizer plants or fish processing know that stench means serious presence, and most aren’t thrilled to hang out in it. But what about the concern behind the aroma?
Daily life barely bumps into trimethylamine unless people work in certain jobs. Chemists use it, industries create it as a byproduct, waste-treatment plants release it, and yes, decaying fish generate a lot. Some folks with rare health conditions even produce extra in their sweat and breath, giving off that unmistakable scent.
Breathing in the fumes turns out to be more than a nose-wrinkler. At low levels, it mostly acts as a nuisance. At higher concentrations, things shift. Eyes and nose may sting, throats itch, and some break out in coughs. Research by OSHA and the CDC points out that exposure to air thick with trimethylamine can irritate lungs and eyes, sometimes enough to send workers to the doctor. Nausea and headaches join the list with repeated or heavy exposures. One study I remember showed that 100 parts per million brings pretty strong symptoms fast.
Touch brings another kind of trouble. Trimethylamine doesn’t just vanish after making contact. It can cause rash or burns if it’s concentrated, especially with repeated unprotected handling. Spills in water don’t last, since this chemical evaporates quickly, but if someone drinks water tainted with it, gut upset usually follows—not life-threatening, but not pleasant either. It won’t build up in your body from the environment, but direct contact shouldn’t be taken lightly. Nit-picking over regulations, the EPA sets some numbers for what counts as “safe”—and that’s for a reason.
In the real world, trimethylamine rarely causes emergencies outside workplaces or factories. Emergency rooms don’t report people flooding in with poisoning cases, yet persistent irritation and health complaints from people around industrial sites can add up. I remember a friend who worked in wastewater by day and started struggling with dry, red eyes and scratchy skin. Turning up the ventilation and using goggles put a stop to the worst symptoms for them. Seems simple, but not everyone gets the basics in place.
Factories and treatment plants that protect workers tell a better story. Routine mask-wearing, fresh air ventilation, and fast cleanup for spills take the bite out of exposure. Small changes like these cut health problems. On a bigger scale, stricter air-quality checks and early warning sensors flag big leaks before they spread through offices or neighborhoods. Offices and communities near big producers benefit from clear info, regular air checks, and honest reporting from companies.
At home, the odds of running into trimethylamine at dangerous levels stay tiny. Keeping drinking water safe and supporting basic air-quality measures keeps this foul chemical from turning up where it shouldn’t. For most, encountering it stays a brief, smelly story—not a toxic disaster.
People working with chemicals learn quickly which ones can turn a calm shift into a fire drill. Trimethylamine definitely shows up on that list, mostly thanks to its overpowering fishy smell and its habit of escaping from containers. Labeled as both flammable and toxic, trimethylamine does not belong near food prep, smoking, or open flames. In a plant setting, where distractions pop up all the time, just a whiff tells you someone missed a step somewhere. Most folks don’t realize that a small leak can fill an enclosed area with hazardous vapor before anyone notices.
Standard procedure usually calls for storing trimethylamine in tightly sealed steel drums or approved pressure-rated tanks. Manufacturers prefer drums fitted with proper venting valves to avoid pressure buildup, since this compound feels like making its own exit plan once temperatures rise even a bit. I remember one older warehouse where the drum seals kept failing during summer afternoons; the management had to install better ventilation and add air conditioning to the chemical bay just to keep up.
Some teams make the mistake of storing it with ordinary chemicals, but trimethylamine reacts strongly with acids and can corrode copper and alloy metals on contact. I’ve seen pitted pipes and stained steel sheets from forgotten spills. Experts recommend painted or epoxy-lined containers if the operation calls for any sort of metal transfer. Absorbent pads, not just paper towels, make cleanup safer when someone does end up spilling some.
Trimethylamine starts forming explosive mixtures with air at low concentrations. Spark sources—like a static charge on a work shirt or a phone dropped on the ground—can trigger disaster. Fire suppression shouldn't only include extinguishers; staff need fire blankets and fixed overhead systems in ready reach. At one factory I visited, a minor leak in a pump seal set off alarms, bringing the whole packing room to a halt until hazmat teams confirmed the area was safe.
Vapor monitoring equipment needs to stay calibrated. Too many facilities gamble with old sensors or rely on human noses to detect leaks, but high concentrations can numb the sense of smell. Installing fixed gas detectors with clear alarms, plus training the crew to respond right away, keeps the workplace safe. Monthly drills help cement the right habits, whether it’s getting to fresh air or containing a spill before it spreads.
Safe trimethylamine storage starts with common sense. Place drums in cool, shaded spaces far from welding, battery charging, or anything sparking. Assign a dedicated shelf or closet and post warning labels that nobody can miss. The best-run plants put up clear signage, keep updated safety data sheets close at hand, and rotate stocks so older drums don’t quietly build up pressure in the back of the storeroom.
I’ve seen facilities where safety culture means double-checking each shift and replacing worn seals long before leaks start. Regular training, quick response plans, and a few well-placed environmental controls make all the difference. While paperwork and compliance sound dull, they beat sorting out the aftermath of an accident. This isn’t abstract red tape; people get hurt if nobody pays attention.
Trimethylamine may not make headlines unless something goes wrong, yet the risk is real. For anyone responsible for storage and handling, it never pays to take shortcuts. Tools like self-closing lids, emergency wash stations, and real-time gas monitors aren’t luxuries. Strong habits, sharp tools, and a workplace that values double-checks are the basic ingredients for a safer operation. Those extra steps save lives and keep the workday uneventful—for everyone’s sake.
Trimethylamine shows up in the formula C3H9N. It’s built from three methyl groups, each with a carbon and three hydrogens, linked to a single nitrogen atom. The structure looks kind of like a tripod, with the nitrogen atom right at the center, holding each methyl group at arm’s length. Forget about fancy chemical diagrams—picture ammonia, but swap out the hydrogens for little carbon-based arms swinging off the nitrogen.
This little molecule has a reputation. If you’ve ever caught a whiff of rotting fish or left an open can of seafood in the sun, then you’ve met trimethylamine’s signature smell. It lingers in kitchens, fish markets, and even certain cleaning agents. The reason it packs such a punch comes down to its small size and volatility—once exposed, it escapes right into the air and straight into your nose.
Most people bump into trimethylamine long before they care about its chemical formula. Some of my most vivid childhood memories come from watching older family members clean fish at a lakeside cabin, the air heavy with the unmistakable scent. Looking back, it’s wild how a single chemical, so tiny, can instantly mark a place and moment. In biology, trimethylamine forms in our bodies, too. Certain gut bacteria break down foods rich in choline—think eggs, legumes, and even some meats—releasing trimethylamine as a byproduct.
For some, this process becomes a real problem. Take trimethylaminuria, sometimes called “fish odor syndrome.” Folks with this rare disorder can’t convert trimethylamine into a more odorless molecule, so it builds up. The result: an unavoidable, persistent fish-like body odor. This isn’t about hygiene—diet, genetics, and even stress play roles. The stigma attached to something no one can control is something society rarely stops to consider.
Trimethylamine isn’t just an oddity of biology or an olfactory hazard. The chemical gets used in important areas—production of certain medications, agricultural chemicals, and water treatment processes. In the lab, manufacturers count on its ability to act as a building block. It shows how chemicals with unpleasant reputations still serve a purpose most folks rarely acknowledge.
On the flip side, industrial release of trimethylamine raises questions about air quality. Communities living near fish processing plants, chemical factories, or waste facilities can struggle with persistent odors. Long-term exposure can even irritate eyes or skin. Regulators and engineers need to balance usefulness with public well-being. Proper ventilation, scrubbing systems, and containment are real solutions—not distant dreams.
So, trimethylamine’s structure—N with three CH3 groups—makes it more than just a textbook curiosity. The reality of its chemistry plays out in riverside seafood markets, family kitchens, and high-tech manufacturing. If conversations about chemicals like trimethylamine become less clinical and more grounded, maybe we start treating both the science and its impacts with the respect they deserve.
Trimethylamine isn’t a household name, but it gets remembered for its stink. Even a small spill brings a strong odor, enough to turn heads and spark a panic. Beyond the smell, the chemical causes eye, skin, and lung irritation. For folks working in labs, food factories, or water treatment, dealing with such spills isn’t rare—mistakes happen. Delays or sloppy cleanup create bigger headaches, and medical bills rise fast if exposure gets ignored.
Trimethylamine shines as a reminder of why training matters. Breathing its vapors pulls a burning feeling into the chest, and redness shows up quick where skin touches the liquid. The Environmental Protection Agency pays attention because the fumes build up fast, especially in closed spaces, and can spark fires if an open flame is nearby.
In my time volunteering with student chemistry workshops, even the smallest mistake handling amines triggered confusion and raced people out of a room. That memory sticks—everybody thinks nothing will go wrong until it does.
Once a spill hits, delaying isn’t an option. Air needs to start moving. Doors and windows stay wide open, and anyone not wearing the right gear steps away. Gloves, goggles, and a protective jacket offer some shield, but a proper full-face respirator keeps exposure down if fumes stay strong. If you grab the wrong gear, a mess can turn personal real quick.
On solid floors, scoops and absorbent pads work. Sand, clay, or commercial spill kits all soak up the mess. Scooping sludge straight into a sealed, labeled container stops the chemical from spreading. Water just spreads the problem further—never hose down a spill. Weak, simple solutions only give room for fumes to build up.
Panic leads to shortcuts, but that only helps chemicals get into the air or into drains where they don’t belong. Never dump trimethylamine in sinks or toilets, no matter how minor the spill looks. It only takes a few moments before harsh fumes fan out across a building.
Fire is a real risk if clean-up skips the basics. Open flames, sparks, and even static electricity from certain tools provide enough heat to ignite vapors. In accident reviews, fire marshals often circle back to small mistakes—missing a spark, not checking ventilation, or hoping that just a towel will do the job.
Nobody loves safety drills, but in real life, practice pays off. Regular training helps everyone learn how to spot chemicals like trimethylamine and know which gear to grab first. Once the spill gets cleaned, the area should always get checked with sensors, not just a sniff test. Emergency plans need to pinpoint who calls for backup—hazmat teams and poison control both know this chemical by sight and smell.
The best defense sticks to simple rules: focus, good air, working equipment, and easy-to-follow plans. Responsible teams don’t cut corners or throw luck at the problem. Whether handling a large tank or a tiny bottle, steady routines shape safer outcomes—and help make sure everyone goes home at night with just a story, not an injury.
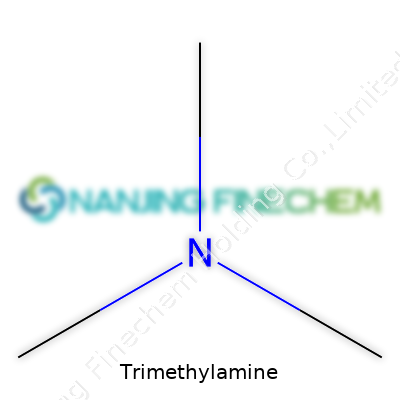
| Names | |
| Preferred IUPAC name | N,N-dimethylmethanamine |
| Other names |
N,N-Dimethylmethanamine TMA Trimethylamine anhydrous Trimethylammonia N-Trimethylamine |
| Pronunciation | /traɪˈmiːθɪl.əˌmiːn/ |
| Identifiers | |
| CAS Number | 75-50-3 |
| Beilstein Reference | 1718730 |
| ChEBI | CHEBI:18139 |
| ChEMBL | CHEMBL1409 |
| ChemSpider | 564 |
| DrugBank | DB01847 |
| ECHA InfoCard | 100.000.012 |
| EC Number | EC 200-875-0 |
| Gmelin Reference | Gmelin 827 |
| KEGG | C00440 |
| MeSH | D014254 |
| PubChem CID | 1146 |
| RTECS number | **WN5420000** |
| UNII | 0E5A2VMB4E |
| UN number | 1083 |
| CompTox Dashboard (EPA) | DTXSID8020217 |
| Properties | |
| Chemical formula | C3H9N |
| Molar mass | 59.11 g/mol |
| Appearance | Colorless to yellowish liquid with a fishy ammoniacal odor |
| Odor | fishy |
| Density | 0.646 g/cm³ |
| Solubility in water | Very soluble |
| log P | 0.16 |
| Vapor pressure | 5570 mmHg (20 °C) |
| Acidity (pKa) | 9.8 |
| Basicity (pKb) | 4.19 |
| Magnetic susceptibility (χ) | -15.9·10⁻⁶ |
| Refractive index (nD) | 1.366 |
| Viscosity | 0.23 cP |
| Dipole moment | 0.59 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 198.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -81.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2056 kJ mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS04, GHS05, GHS06 |
| Pictograms | GHS02,GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H220, H226, H280, H301, H311, H331, H314 |
| Precautionary statements | P210, P260, P271, P280, P301+P310, P305+P351+P338, P304+P340, P312, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 3-4-2-A |
| Flash point | -37 °C |
| Autoignition temperature | 430 °C (806 °F; 703 K) |
| Explosive limits | 2.0% - 11.0% |
| Lethal dose or concentration | LDLo oral human 100 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Trimethylamine: "60 mg/kg (rat, oral) |
| NIOSH | NIOSH: KQ9125000 |
| PEL (Permissible) | 25 ppm |
| REL (Recommended) | 25 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Methylamine Dimethylamine Triethylamine Tetramethylammonium ion Choline Tetraethylammonium ion |