Tetraaminophthalonitrile didn’t get its start in the mainstream conversation. Chemists hunting for specialty ligands and organic synthons kept it mostly within academic circles for years. In the mid-20th century, when researchers were probing new families of phthalocyanines and related compounds, they landed on the nitrile-amine combination as a promising building block. PatENTS started popping up, especially out of Europe and Japan, hinting that commercial interests recognized untapped utility beyond basic dye chemistry and coordination complexes. Despite that, scale-up stayed mostly in university and specialized contract labs, not on the radar of the general public or broad industrial user.
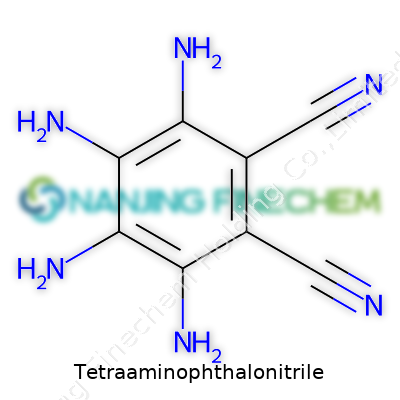
Tetraaminophthalonitrile looks plain but owns a distinctive set of chemical handholds: two amino and two nitrile groups sticking out from a single aromatic ring. Most chemical catalogs list it as a crystalline powder, often pale beige or off-white, though the actual tint shifts a little from batch to batch. Its chemical structure positions it as a prime starting point for crafting products from organometallic complexes to electronics precursors. Many suppliers treat it like a specialty good: firmly aimed at research and development, not the mass-market base.
The compound’s melting point hovers around 255–259°C. Slight solubility in water, better in polar aprotic solvents, and a decent shelf-life—provided you steer clear of high humidity. The aromatic system sits stable, but the nitrile and amino arms make it reactive enough for most seasoned chemists to see big possibilities. Density lands at about 1.42 g/cm³, giving it a substantial feel when handling. On exposure to air, it holds up pretty well, though over months any open container takes on some clumping—the usual fate of any amine-rich aromatic. Its stability under heat and gentle moisture makes it compatible with many processing steps in materials synthesis.
Suppliers typically label it as C8H6N6 with a molar mass of 198.18 g/mol. Purity for most commercial product sits in the 98%+ range, but contract labs sometimes push that higher for semiconductor exploration. Tech sheets set safe temperature guidelines, but I’ve seen working groups push the envelope in controlled inert-atmosphere setups. Companies in the EU and Asia require GHS labeling, with warnings for eye and skin irritation. Most reputable suppliers offer detailed spectral data—NMR, MS, and IR scans—so buyers can confirm identity and look for nagging contaminants. That goes a long way to build trust in a market that sometimes feels like the Wild West.
Synthesis does not require rare precursors or high-pressure reactors. Amination of a phthalonitrile backbone often starts with catalytic hydrogenation or a nucleophilic aromatic substitution; process details get guarded for trade reasons. I’ve seen variants with copper or nickel catalysts and solvents ranging from DMF to DMSO, depending on yield targets and downstream uses. Chemists running custom syntheses emphasize slow addition of ammonia or primary amines to keep side products low. Filtration and recrystallization follow, usually from ethanol or methanol. Some larger labs now experiment with solid-phase supported synthesis to reduce waste and drive green chemistry points.
The twin nitrile and amino groups open the door to a labyrinth of modifications. I once saw a team extend the ring system by reacting it with aldehydes to turn out novel phthalocyanine derivatives—useful for dyes and even organic semiconductors. Its reactivity with metal ions lets it form complexes used in catalysis, sensors, and even certain types of batteries. The amines act as hooks for further alkylation, acetylation, or even polymerization. Downstream, those routes support both new materials and the fine-tuning of electrical properties, which signals opportunity for electronics researchers trying to get more from current designs.
This chemical hides behind more aliases than most would expect. Typical synonyms include 2,3,9,10-Tetraaminophthalonitrile and TApN. Some catalogs simplify things as “tetraamino PN”, but academic publications stick with IUPAC where they can. I keep a notebook with comparative names because even the best scientists can lose track when dashing between supplier catalogs and journal articles. On top of that, some suppliers list it under older naming conventions, especially in translated documentation. Sorting these synonyms out still trips up new students hunting for literature.
Working with tetraaminophthalonitrile never counts as routine. Many labs require full gloves and goggles, even though acute hazards rate as moderate compared to common solvents or strong bases. Direct contact can aggravate skin and mucous membranes, especially over prolonged handling or high concentrations. Its dust, like any aromatic amine, shouldn’t be inhaled. Most facilities run it inside standard fume hoods, with clear labeling on storage jars and regular inventory checks. Waste goes in designated organic bins, not down the drain. In my experience, reminders posted around the lab and regular safety briefings go further than thick manuals. Companies supplying it bet on SDS sheets to keep everyone aware. Incidents stay rare so long as staff treat it with the same respect as any lab-scale specialty chemical.
Despite its low profile, tetraaminophthalonitrile bridges several fields. Materials scientists grab it for phthalocyanine dyes, which run the show in pigments and specialty inks. Its complexation ability means it finds a use in electrocatalysts, where stability and reactivity co-exist. I’ve seen research teams in organic electronics dig into it as a monomer for conductive polymers, aiming at solar cell layers and flexible electronics. Certain pharmaceutical groups investigate derivatives for enzyme inhibition or even anti-cancer pathways. Even battery developers touch it for electrode research. The compound’s batch-to-batch reliability draws niche users, even if it rarely headlines as the core ingredient in major consumer goods.
R&D has blossomed in fits and starts. Grant money rolls in large volumes for organic electronics, and tetraaminophthalonitrile finds itself front and center in some experimental semiconductors. Universities lead in screening new phthalocyanine complexes, testing their magnetic and conductive properties. Research consortia in Europe support green synthesis methods, looking to cut harmful intermediates. Collaboration between synthetic chemists and industrial labs picks up wherever new functional dyes promise to break old efficiency records. The slow pace from bench to industry owes partly to the compound’s limited availability, but open-access publications continue to nudge progress forward year after year.
Long-term studies hover at the edges, but what is known makes sense. Rodent assays peg median lethal doses in the several-hundred-milligram-per-kilogram range, putting it in a similar category to other aromatic amines. No big signals for carcinogenicity yet, but nobody discounts the risk. Regular handling brings up minor irritation more than serious events. Environmental analysts highlight a need for better waste minimization, especially where traces show up in facility outflows. Calls for comprehensive chronic exposure data persist, given that other phthalic derivatives have checked in with worrying results in the past. Until regulators or big review groups push for more, the compound lives in a gray safety zone.
There’s real promise on the horizon for tetraaminophthalonitrile. As labs chase new organic electronics and dyes for greener industries, this compound sits ready as a starting point. Scale-up remains a challenge, both in cost and in waste control. If manufacturers put real muscle behind greener synthesis and high-purity bulk batches, I see a day when it leaves the specialty shelf and enters broader industrial workflows. Cross-disciplinary teams working on electronics, energy storage, and medical diagnostics will keep finding uses for this underdog compound. While the history of synthetic organics shows no guaranteed route to commercial stardom, tetraaminophthalonitrile feels overdue for a broader turn in the spotlight—provided researchers, regulators, and suppliers link arms to address its challenges head-on.
Anyone who’s ever poked through a chemistry textbook knows molecules don’t have to fill half the page to matter. Some of the most influential ones look a little unassuming. Tetraaminophthalonitrile is a case in point. Sitting in the family tree that leads to powerful substances like phthalocyanines, this molecule catches the eye of chemists hunting for certain building blocks.
You won’t find this compound on the shelf at your local pharmacy. The structure counts as more than just a mouthful: two benzene rings fused together (a phthalic core), holding tight to two nitrile (C≡N) groups at opposite points, and then not just one or two—four amino (NH2) groups hanging off the remaining corners. Written out, its chemical formula shows as C8H6N6. Look at the ring like a clock: nitriles stick out at the 1 and 8 spots, amino arms wave from 2, 3, 6 and 7.
This molecule wasn’t just dreamed up during a slow day in the lab. In the real world, that arrangement—cyano and amino groups paired together on a tough aromatic ring—opens up a world of reactions. People first meet it while piecing together phthalocyanines, those blue-green dyes that sneak into ink cartridges, car paints, even certain types of solar cells. A simple switch or tweak in the side groups can nudge a molecule’s behavior in a new direction, and it’s these little changes that drive progress in chemical manufacturing.
Four amino groups means four jumping-off points for further chemical work. They act like sticky hooks, ready to grab onto metals or slip into bigger frameworks. This becomes important for more than laboratory curiosity: phthalocyanines have made their mark in medical imaging, light capture, and as catalysts doing heavy lifting in industry.
Chemists can imagine beautiful molecules on paper, but real life asks more. Getting something as packed as tetraaminophthalonitrile to behave is a constant challenge. The molecule doesn’t take kindly to rough handling. Those amino arms bring welcome utility, but they also mean the compound needs protection from the wrong solvents and temperatures, or you’re left with a mess instead of a tool.
Factories struggle with synthesizing molecules like this at large scale. The reactants often don’t play nice. The byproducts clog up filters. In university settings, enough patience and time can finesse a product into a test tube, but safe, affordable bulk production still presents a host of headaches. Environmental safety and worker protection get tangled up with cost and purity targets.
Looking at the synthesis problems, a lot rests on better reaction control. Green chemistry stands as more than a buzzword — by tailoring steps and scouting for safer solvents, labs start to shrink toxic waste. Automated flow reactors show some promise, dialing in heat and mixing so reactions don’t run off the rails. Open sharing of improved lab methods speeds up breakthroughs, too. In the wider world, researchers can work with industry to swap out legacy recipes and update protocols, especially when the payoff is a more stable or cleaner product.
People might never recognize tetraaminophthalonitrile by name. Yet every time they use something colored by phthalocyanines—or rely on a specialty material that owes its strengths to a few well-placed amino and nitrile groups—they benefit from chemists who see value in small structures. Tools like this don’t make front-page news, but they give the world brighter colors, more efficient energy, and the roots from which innovation grows.
If you’ve spent some time around a university chemistry lab or read up on specialty chemicals in electronics, Tetraaminophthalonitrile—sometimes shortened to TAPN—crops up as one of those mouthfuls that people in white coats perk up about. It hasn’t found its way into household conversation, but dig a little deeper and TAPN’s fingerprints show up in places many wouldn’t expect.
Chemists see TAPN more as a stepping stone than a final destination. With four amine groups and two nitrile groups sticking off the same ring, this molecule is practically calling out to be turned into something else. The nitrile groups hold on tight to their electrons, making them ready for reactions that spin out all sorts of specialty compounds. Society wouldn’t have a rainbow palette of dyes or tough materials in circuit boards without intermediates like this one.
In the dye industry, TAPN rides shotgun in the creation of phthalocyanine pigments. I remember a summer working through a stack of technical papers on bright blue lake pigments—the sort of blue that pops up in packaging ink or anti-counterfeiting marks. That intense blue color traces part of its chemical genealogy back to molecules like TAPN. Unlike other chemicals that fade or give up over time, phthalocyanine pigments stay strong and reliable, and TAPN gets some of the credit for making those connections happen.
Ask around in the electronics world, and you’ll catch references to TAPN as an intermediate in making semiconducting polymers and liquid crystals. Devices like organic LEDs or sensors hiding inside everyday gadgets depend on rigorous molecular design. TAPN supplies the framework chemists use to build highly specialized electronic materials that work in all sorts of temperatures and conditions.
Organic semiconductors aren’t science fiction anymore. TAPN’s role might not make headlines, but the tiny improvements it helps enable ripple out in the form of faster phones, sharper screens, and flexible electronics. It’s easy to overlook the value of a molecule that disappears during the reaction, yet the discipline it brings to molecular architecture underpins the reliability that tech companies demand.
I once visited a conference booth packed with lab samples that looked like colored glass shards—advanced polymers engineered for extreme environments. The company quietly referenced TAPN in a few of their technical notes. Turns out, incorporating tetraamino units builds sturdier links in the polymer backbone, adding just the right combination of stiffness and flexibility. This is where chemical intuition—marrying raw experimentation with years of theory—helps innovators squeeze more performance from less material.
TAPN doesn’t grab the limelight like flashy innovations, but its practical footprint is everywhere. As materials research swings toward sustainability and efficiency, zeroing in on intermediates with multiple reactive sites makes sense. By expanding the catalog of what these simple building blocks can do, we end up with stronger, lighter, and smarter materials, from medical devices to solar panels.
TAPN is not a household name, yet. But step behind the scenes in laboratories or sit in on the right research meeting, and you’ll see how much of modern life leans on modest, reliable building blocks like this. It’s a reminder of how much daily progress happens not in radical shifts, but in careful, incremental tweaks to hidden chemistry. Putting the right molecule in the right place sends ripples far beyond the beaker, all the way to products in our pockets and homes.
If you’ve worked with specialty chemicals like tetraaminophthalonitrile, you know storage isn’t just a detail buried in paperwork. This compound plays a role in organic electronics and advanced materials, so a small mistake with storage can cause headaches across the lab. Getting storage right keeps lab workers safe, keeps experiments on track, and stops contamination before it starts.
Tetraaminophthalonitrile doesn’t explode at the drop of a hat, but it doesn’t thank you for being careless either. Many amine-containing compounds react strongly with acids, oxidizers, or even a bit of stray moisture. Humidity can creep into containers, break down the sensitive structure, or even trigger unexpected reactions with the atmosphere. People returning to ruined samples or, worse, labs filled with strange fumes already know what careless storage does to research and budgets.
Packing this chemical away calls for a cool, dry, and dark environment. Think temperature-controlled cabinets or refrigerators set between 2°C and 8°C. Heat speeds up decomposition, so room temperature doesn’t cut it for long-term safety. Moisture is just as bad. A desiccator or a cabinet with built-in drying packets helps a lot, especially in humid climates where even tightly closed bottles pull in water from the air. Light isn’t a friend either—UV rays and certain wavelengths degrade the compound or spark messy by-products. Amber vials and opaque cabinets keep light out and give extra peace of mind.
Glass vials or bottles with air-tight, chemical-resistant seals stand up to reactions and leaks better than plastic containers. Most laboratories stick with borosilicate glass and use caps fitted with PTFE (Teflon) liners. It only takes one cheap plastic lid to let in air, so investing in proper supplies pays long-term rewards. Labels wear off, so I always double-bag samples, using clear but sturdy zip bags and writing the storage date big and bold.
Crowding chemicals in one cabinet leads to problems down the line. Keep tetraaminophthalonitrile away from acids, peroxides, or oxidizers. Every chemical inventory I’ve built includes a quick-reference chart to separate amines from the troublemakers nearby. Simple habits like not storing incompatible chemicals together prevent hours of cleanup and financial waste later on.
Spills can turn a safe lab into an unsafe mess. This compound isn’t highly toxic by every measure, but dust and particles go airborne with just a brush or spill. Gloves, goggles, and a well-fitted mask protect anyone who’s handling the jar or transferring solids. Wiping surfaces with a damp towel and disposing of all cleanup materials in a clearly labeled chemical waste bin keeps the risk low.
I’ve run enough chemical storerooms to know that training staff and students makes or breaks safety efforts. One quick walkthrough pointing out hazards, demonstrating how to handle containers, and showing the results of poor storage decisions always gets the message across. Scheduling regular checks of storage areas keeps reminders fresh and stops problems before they grow. Clear, shared protocols become the backbone of safe chemical handling—no matter how rare or advanced the material in question.
Tetraaminophthalonitrile sounds like something you’d find in an intimidating lab, surrounded by blinking lights and scientists in full Hazmat gear. In reality, it isn’t as well-known as more common industrial chemicals, but some key facts about its safety help show why a little caution goes a long way—even with the complex stuff.
Chemists will tell you that a nitrile group, like the one in this compound, often brings up concerns about toxicity. I’ve handled nitriles in the lab before, carefully watching for fumes that might irritate eyes, lungs, or skin. Tetraaminophthalonitrile takes a spot in the family of phthalonitriles—a group used in dyes, pigments, and sometimes electronic materials. Most similar chemicals ask for basic safety measures: gloves, a good lab coat, goggles to catch the splashes or dust, and plenty of airflow. No need for moon suits, but nobody shakes out nitrile dust with bare hands.
I’ve met people who roll their eyes at safety warnings because they seem like overkill. After all, nothing exploded, right? But I’ve learned that repeated, small exposures turn into a real problem. Skin irritation or a cough today grows into a doctor visit tomorrow. Nitriles can be absorbed through the skin or inhaled as powder. The amine groups in tetraaminophthalonitrile also bring a bite; direct contact irritates, and the chemical’s structure means it might break down into nastier stuff if heated to high temperatures or mixed with certain acids or oxidizers. No need to panic, but if my shirt caught a fine dusting of the substance, I’d head for the nearest shower in a hurry.
Tetraaminophthalonitrile doesn’t appear on every regulatory list, so you won’t always find a stack of reports about it on the internet. But it pays to read the label, even if it’s tempting to trust a coworker’s advice. Globally Harmonized System labels tend to list “harmful if inhaled or swallowed” or “causes skin and eye irritation” with many similar materials. The best approach? Don’t eat lunch right after handling this powder, no matter how careful you think you’ve been.
Lab culture sets the tone. I worked in one place where everyone took glove use seriously: people checked each other’s old habits as quickly as they checked their work. That encouraged listening to gut feelings, washing hands an extra time, and speaking up about spills. Tetraaminophthalonitrile wouldn’t cause mayhem if you treat it with respect, the same as strong cleaning agents or paints. But complacency invites slips.
It doesn’t take complicated gear to stay safe with tetraaminophthalonitrile. Ventilation, gloves, goggles, a long-sleeved layer, and hand-washing catch most threats. Training new folks to spot trouble and not cut corners saves more problems than any warning label. Tracking small exposures, quick cleanups, and keeping a clear line between the chemical area and your breakroom beats regret any day.
Anyone searching for reliable Tetraaminophthalonitrile will notice plenty of suppliers wave around purity specs, usually boasting a figure above 98%. A high number like that might relax a few nerves, but it’s useful to look past marketing jargon to see what that figure means for real-world lab work or industrial production.
I’ve seen more than my share of production setbacks linked to unclear or misleading purity claims. Purity, in plain terms, indicates the percentage of the material that is truly Tetraaminophthalonitrile, not unidentified contaminants or leftover reagents. If a batch only hovers around 95%, mystery by-products could show up during synthesis, pushing up purification costs or clogging up test results.
Reputable suppliers back up their claims with detailed documentation. That means a Certificate of Analysis should arrive with every shipment, listing actual test results—things like HPLC, NMR, or even elemental analysis. These reports break down known side products and the actual percentage of the main compound, not just a generic “99% pure” stamped on a label.
My own routine involves comparing this documentation against independent tests, if stakes are high or the end application demands strict purity. I’ve caught batches where the listed purity seriously stretched the truth. Especially with less common chemicals, trust only goes as far as verifiable facts.
Manufacturing Tetraaminophthalonitrile isn’t simple. Reactor conditions, raw feedstock, even the way workers handle solvents can introduce traces of unwanted chemicals. Sometimes a reaction leaves behind close cousins of the desired molecule. Other times, solvents stick around if the factory rushes drying or doesn’t use proper vacuum equipment. If the supplier skimps on post-reaction cleanup, those tiny leftovers pile into the finished material.
Each impurity has consequences. In high-purity applications, undetected trace contaminants can disrupt sensitive reactions or put a hitch in analytical work. For industrial scale-up, accumulating impurities complicate downstream purification, chewing into budgets and time. I’ve watched entire days go sideways because unlisted trace solvents gummed up a chromatographic column or threw off product yield in a pilot run.
Choosing a Tetraaminophthalonitrile source isn’t only about finding a big purity number. Batch consistency comes up just as important—I look for a supplier with a track record of sending the same quality material year-round, not just one lucky batch. Samples from multiple lots, tested independently, make or break supplier relationships in my experience.
There’s also the question of what fits the final project. For R&D or exploratory work, slightly lower purity could work if the price is right and you plan to clean up material yourself. For regulatory filings, any quirks or contaminants not already vetted risk halting progress or raising eyebrows during audits. I’ve watched teams lose months chasing down unidentified peaks in analysis, just because chemical supply cut corners.
Getting clear answers from vendors goes a long way. I always ask for batch-specific COAs, not generic examples. Direct conversations about process controls, cleaning steps, and packaging conditions cut through lots of guesswork. If a supplier won’t show their cards, it’s a safe bet their product won’t make the grade.
In my line of work, high-quality supply chains build on trust, transparency, and proof—not just numbers printed on a spec sheet. Anyone who depends on Tetraaminophthalonitrile for serious work knows those details keep things running smoothly from synthesis to final results.
| Names | |
| Preferred IUPAC name | 2,3,9,10-Tetraazanaphthalene-1,4-dicarbonitrile |
| Other names |
TAPN 1,2,4,5-Tetraaminobenzene-3,6-dicarbonitrile |
| Pronunciation | /ˌtɛtrə.əˌmiːn.oʊˌθæloʊˈnaɪ.trɪl/ |
| Identifiers | |
| CAS Number | 2909-47-7 |
| Beilstein Reference | 3532081 |
| ChEBI | CHEBI:52725 |
| ChEMBL | CHEMBL2017569 |
| ChemSpider | 20266359 |
| DrugBank | DB07780 |
| ECHA InfoCard | 03e6e5b4-c73d-4a6b-966c-cbc1755b3bfa |
| EC Number | EC 246-680-4 |
| Gmelin Reference | 155078 |
| KEGG | C11131 |
| MeSH | D017974 |
| PubChem CID | 151333 |
| RTECS number | SR1050000 |
| UNII | SRQ0479D2X |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID4046502 |
| Properties | |
| Chemical formula | C8H6N6 |
| Molar mass | 288.28 g/mol |
| Appearance | Off-white solid |
| Odor | Odorless |
| Density | 1.36 g/cm3 |
| Solubility in water | Insoluble |
| log P | 0.22 |
| Acidity (pKa) | 26.1 |
| Basicity (pKb) | 3.66 |
| Magnetic susceptibility (χ) | -0.0000116 |
| Refractive index (nD) | 1.720 |
| Dipole moment | 1.03 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 303.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -51.8 kJ mol-1 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| LD50 (median dose) | LD50: > 2000 mg/kg (rat, oral) |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 50 μg/m³ |
| Related compounds | |
| Related compounds |
Phthalonitrile Tetranitrophthalonitrile Tetrabromophthalonitrile Tetracyanophthalocyanine 1,2,4,5-Benzenetetraamine Phthalocyanine |