Pregnane-3,20-dione, known to many as progesterone, arrived on the scientific scene through a series of discoveries in the early twentieth century. As chemists cracked open the secrets of steroid hormones, this molecule slowly drew attention for its role in reproductive health. Early isolation techniques relied heavily on animal tissues, mainly from corpora lutea, which weren’t efficient or scalable. The breakthroughs came with advancements in organic synthesis, turning tedious extraction into laboratory precision. By the 1940s, researchers pushed boundaries to make large-scale production a reality, notably with the work of Russell Marker and his development of the Marker Degradation using plant steroids from Mexican yams. This achievement shifted progesterone from an obscure biological curiosity into a compound with economic and clinical significance.
Today, pregnane-3,20-dione stands as a staple for hormone therapies, both in medicine and agriculture. Its applications reach far beyond its birth in reproductive research. You might find this compound as an active ingredient in hormone replacement therapies, contraceptives, and veterinary solutions. Large pharmaceutical companies make sure physicians and researchers have access to this molecule in pure form, while regulatory agencies keep an eagle eye on its quality and sources. The demand never really dips, as evolving roles in new therapies—as an anti-inflammatory agent or even as a neurological stabilizer—keep bringing fresh relevance. I’ve noticed that everytime science pushes deeper into steroid biochemistry, it almost always circles back to this gritty, versatile molecule.
Pregnane-3,20-dione displays a pale, odorless, crystalline form at room temperature. Its structure packs a solid molecular punch: C21H30O2. Melting occurs near 126-130°C, usually without decomposition, which signals decent stability during processing. This steroid doesn’t dissolve well in water, but mix it with ethanol, chloroform, or other organic solvents, and it goes right in. The molecular backbone itself offers a rigid three-dimensional shape—a characteristic helping it slot snugly into hormone receptors. Such chemical stubbornness gives it an edge during extraction and formulation; it’s not easy to degrade or tamper by careless handling, so I’d say it serves well in the real world of pharmacy and research labs.
Pharmaceutical-grade batches demand over 99% purity, with minute residual solvents and defined particle sizes to support uniformity in dosing. Labels on commercial progesterone speak clearly to batch numbers, production and expiration dates, and solvent profiles. Safety data sheets, even for small vials, closely record the substance’s CAS number (57-83-0), weight, storage requirements, and transport regulations. Shelf lives generally run several years under controlled, dark, cool storage. Technical specs don’t only serve regulatory boxes; clinical outcomes depend on reliable potency, so anyone in a lab or pharmacy needs clear assurance that the compound comes uncontaminated and traceable straight to the source.
Classic production approaches kick off with plant steroids like diosgenin, which comes from wild yams or certain types of tubers. Chemical transforms—hydrolysis, oxidation, reduction—guide the plant molecule stepwise towards pregnane-3,20-dione, bypassing tricky steps in animal extraction. For those who love organic chemistry, this synthesis brings satisfaction: multi-stage, precise, and often improved by green chemistry tweaks over the years. More modern biotechnological routes now use engineered microbes to crank out sterol intermediates, refining production and lowering cost. Scale-up remains a balancing act of yield, purity, and responsible waste management, but I’ve seen that commitment to greener, safer methods only grows with each decade.
Chemists see pregnane-3,20-dione as a launching pad for more complex steroids. Common reactions involve reduction of the ketone groups, halogenation on the A or D rings, or introduction of oxygenated side chains. Medicinal chemists often tweak the basic ring to engineer new pharmaceutical agents, especially for anti-inflammatory or contraceptive use. These changes not only create drugs with tailored biological activities, but often sidestep patents, stimulate competition, and push the boundaries of pharmacology. From the workshops of mid-century pioneers to today’s high-throughput screening labs, this molecule refuses to sit quietly. Each fresh reaction carves out new opportunities.
Pregnane-3,20-dione travels under several names: progesterone, corpormon, luteal hormone, and progestin, just to name a few. In documentation, you’ll see registry identifiers like UNII: 4G7DS2Q64Y or PubChem: 5994. Pharmaceutical companies stick to recognizable synonyms to avoid confusion; some generics brand it with catchy names for consumer access, while chemical catalogs and academic papers tend to use the shortest possible reference. In practice, a clear chain of synonyms ensures researchers and prescribers don’t trip over paperwork or supply chains. Even so, mislabeling still crops up—so a careful check always pays off.
Anyone handling pregnane-3,20-dione should wear gloves and goggles, respect ventilation guidelines, and aim to prevent unnecessary contact. Material safety data lists mark it as low-to-moderate in terms of toxicity, with inhalation and prolonged skin exposure best avoided. Chronic high-dose exposure triggers changes in endocrine function, so occupational settings must respect exposure thresholds and regular training. Storage in cool, dry spaces away from direct sunlight keeps the compound robust and delays breakdown. Disposal procedures line up under hazardous chemical waste, no shortcuts. Regulation hasn’t slackened, either: Good Manufacturing Practice (GMP) protocols and ISO certifications secure standards on every step. For anyone in labs—not just chemists but also janitors and logistics staff—real-world vigilance avoids the sort of incident that makes headlines.
Doctors use pregnane-3,20-dione for a spread of conditions: supporting pregnancy in IVF, curbing heavy menstrual bleeding, and treating some forms of endometrial cancer. It also underpins the market for combination oral contraceptives. In animal husbandry, it helps regulate breeding cycles and brings predictability to agricultural planning. Neuroscience labs dig deeper each year, hoping to pin down its role in brain injury recovery and mood regulation. Through all of medicine’s changing seasons, this molecule’s basic profile lets it branch into unexpected territory—proof that solid chemical roots can yield surprising fruit.
Recent research turns its gaze toward designing novel analogues with improved selectivity or reduced side effects. Labs experiment with oral and transdermal formulations to overcome metabolism challenges that break down the hormone too quickly. There’s a push to harness this basic structure for anti-cancer agents and immunomodulators, blending steroid backbone with innovative functional groups. At the same time, researchers look for plant- and microbe-based production for better sustainability. Collaborations among chemists, biologists, and engineers have fueled grant proposals and sparked small startups aiming to shake up production efficiency. My own time in academic labs showed me that progress never moves in straight lines; setbacks on the bench often light the spark for the next big leap.
Toxicology reviews show that, at therapeutic doses, pregnane-3,20-dione remains safe and well-tolerated. Problems usually show up with overdosing or long-term misuse: liver strain, electrolyte loss, weight changes, and in rare cases, blood clots. Reproductive and developmental toxicity gets top scrutiny, especially in veterinary and agricultural applications. Regulatory agencies set permissible residue limits in food to keep consumer risk in check. Environmental studies weigh the persistence of synthetic progestins in water supplies, pushing for biodegradable alternatives and stricter effluent controls. Toxicity research isn’t static; as new analogues reach preclinical studies, risk profiles are reassessed and sometimes rewritten, shaped by better technology and more nuanced understanding.
Looking forward, pregnane-3,20-dione sits at the crossroads of pharmaceutical innovation and green chemistry. Solving longstanding supply and waste problems calls for smarter, cleaner synthesis, as well as microbial factories that turn low-value feedstock into high-purity product. Advances in controlled delivery systems—like slow-release implants and designer nanoparticles—could unlock new therapies for chronic illness, shifting treatment from daily pills to once-a-season injections. As neuroscience opens further, potential links between this hormone and brain health might redraw boundaries for both therapies and diagnostics. Science and industry can’t afford to rest easy with legacy routes and applications. With tighter environmental rules, patient-centered care models, and the relentless march of unmet medical need, pregnane-3,20-dione’s next act won’t repeat the old script. Reactivity, reliability, and resilience define its importance, and the story is far from finished.
Take a look at the chemical’s full name and it sounds like something only a scientist could love. Pregnane-3,20-dione goes by another name that’s far more familiar: progesterone. It’s one of the main hormones running the show in our bodies, particularly in the realm of reproduction and pregnancy. In the pharmacy world, this molecule isn’t just some dusty research compound—it’s a daily reality for folks dealing with a whole range of hormone-related health needs.
The best-known role for pregnane-3,20-dione shows up in women’s health. Doctors rely on it for hormone therapy, whether that’s managing irregular cycles or assisting with symptoms that flare up around menopause. It plays a big role in helping the uterus get ready for pregnancy every month and keeping a potential pregnancy steady in the early weeks. If the body’s own supply drops too soon or never quite gets started, supplements and injections fill that gap. That can make a real difference to folks who want to build a family, particularly with assisted reproduction techniques.
Pregnane-3,20-dione also finds a place in medicine for things way beyond just fertility. Some doctors prescribe it as part of hormone replacement therapy, especially for women who’ve gone through menopause and face night sweats, trouble sleeping, or mood swings. In that setting, it acts as a backup for lost natural hormones and can soften the rollercoaster ride of hormone changes. In a different setting, it helps treat irregular or skipped periods—by acting like the body’s own chemical messenger, it helps restart the cycle. There are even unique uses, like lowering the risk of miscarriage in some pregnancies or managing certain cancers tied to hormones.
Tracing this chemical’s path from lab to pharmacy shelf, I see the direct effect on real people—friends have gone through rounds of hormone shots, co-workers have handled mood swings with medication, family members have counted on hormone pills to get through rough patches. The science works right alongside the messiness of life, which should always shape how we talk about the benefits, the risks, and the cost. Progesterone hits the market in plenty of forms—tablets, skin gels, injections—so access depends on insurance, local pharmacies, and sometimes stubborn supply chain hiccups.
Problems sneak in, too. Overuse and sloppy prescriptions can lead to side effects like weight change, headaches, or spotty moods. And not everyone gets the same results—some patients wrestle with long waits, high prices, or tough side effects. I’ve heard from women who spent months fighting for the right dose or hunting down a brand that doesn’t cause a rash. There’s room for improvement here. Smarter prescription practices, clearer information for patients, and support groups can make a big difference. Research rolls forward, searching for new ways to make hormone therapy safer, easier, and more predictable.
At the end of the day, pregnane-3,20-dione reflects more than a chemical formula. It shows how science and everyday needs collide, and how the details of hormone care ripple out into so many corners of life. Investing time in understanding both the molecule and the people relying on it stands to make every prescription that much more meaningful.
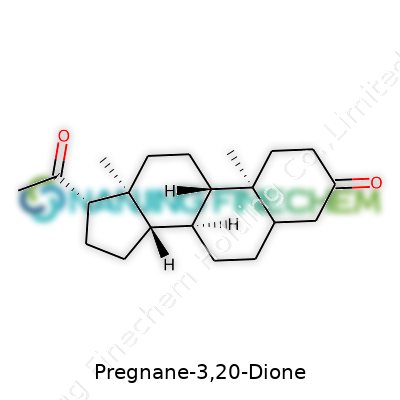
Pregnane-3,20-dione has a sharp, almost clinical ring to it, but under the jargon sits a compound you bump into every time you read up on steroid hormones. Its chemical formula is C21H30O2. This means the molecule includes 21 carbon atoms, 30 hydrogens, and 2 oxygens. Those ingredients build the core skeleton for a variety of steroid hormones people talk about in medicine, sports, and sometimes even in daily health scares.
Zoom in on its skeletal design, and you’ll see four linked carbon rings—three hexagons and a pentagon—forming what chemists call the “pregnane” backbone. If you’ve taken high school biology and remember the basic shape of cholesterol, this will look familiar. Pregnane-3,20-dione, sometimes called “pregnadione,” gets its name because of two defining features: a keto group at the 3rd position and one at the 20th position of the steroid backbone. Both groups house double-bonded oxygen, making it a dione—"di" for two oxygens joining that beefy structure.
Picture the rings like a complex piece of scaffolding—a base that lets life customize something for its needs. The only real difference between pregnane-3,20-dione and other famous steroids, like progesterone, comes from swapping a few functional groups or flipping a bond here and there. It’s this fine tuning that turns a molecular framework into something with potent effects on the body.
Some molecules just tend to show up across pivotal moments in human health. Pregnane-3,20-dione falls into this category. On one hand, it pops up as a metabolic intermediate—you stumble across it as your body breaks down or builds up steroid hormones. If you're looking into progesterone, cortisone, or even testosterone metabolism, this molecule knocks on the door.
Researchers treat pregnane-3,20-dione as a starting point for charting chemical pathways. This compound helps in making synthetic hormones for managing hormonal disorders or easing the symptoms of menopause. Getting a grip on its structure aids in building drugs that balance benefits and avoid harsh side effects.
Tracing the presence and movement of pregnane-3,20-dione gets tangled. Labs face short detection windows. The body can flip and tweak each group on the molecule, making precise tracking a challenge—think playing detective inside a busy cocktail party of similar molecules. Routine tests don’t always pick up these subtle shifts, and results might not show the whole picture of someone’s hormone health.
To sort these hurdles out, smarter detection methods and better laboratory techniques keep coming up. Mass spectrometry, for example, helps discriminate between close chemical siblings. More affordable and reliable assays need to reach smaller clinics and not just top-tier research hospitals. With open data sharing and collaborative research, scientists and doctors edge closer to offering solutions that target the exact hormone pathway tripping people up, instead of throwing a blanket fix that could drive side effects.
Pregnane-3,20-dione might not make headlines often, but its role in health, pharmaceuticals, and research gives it staying power. Keeping an eye on progress in understanding and tracking molecules like this opens the door to better treatments and sharper diagnostic tools. The more we learn about the nuts and bolts, the better we handle hormone-related health challenges tomorrow.
Pregnane-3,20-dione—better known as progesterone’s backbone—shows up wherever scientists dig into steroid biology. It slips into research papers as a “metabolite,” and it gets talked up in labs for its part in hormone pathways. Still, questions about its safety sound important, because the chemical sets off a long chain of biological events, and those can shape more than just test tube results.
Progesterone, the friendlier version of pregnane-3,20-dione, handles some of biology’s biggest jobs: pregnancy, menstrual cycles, even stress response. It calms inflammation and helps regulate blood sugar, but anybody who has lived through puberty or pregnancy knows things get complicated quickly. The body keeps delicate tabs on how much is floating around, and it controls how much gets made and broken down. Adding more—especially from outside sources—rarely plays out smoothly.
Most doctors have worked with progesterone, not pure pregnane-3,20-dione. And the difference matters. Progesterone has decades of trials behind it; patients take it for hormone therapy or birth control. Sometimes they complain about mood swings, headaches, or even blood clots, but millions rely on it anyway. Pregnane-3,20-dione, though, barely gets a mention in clinical guidelines. Limited studies hint that, because it’s part of a natural pathway, the body will turn extra amounts back into things it already uses. No big surprises have shown up in animal studies so far, but those results only go so far. Rats and mice might not share the same weak spots as people do.
I’ve watched researchers get excited over chemicals like pregnane-3,20-dione because they promise insight into rare diseases and hormone disorders. On the flip side, they worry about “off-target” effects. One wrong nudge and all sorts of sensitive feedback loops could tilt out of balance—menstrual cycles could change, mental health could shift, metabolic issues could show up unexpectedly. If the body gets flooded with even seemingly familiar molecules, the usual brakes and checks don’t always kick in. That sort of system chaos can slip under the radar at first, only to show up suddenly for some people. Biosynthetic chemicals leave bigger fingerprints than almost anything we eat or touch every day.
People ask, “Is this safe?” but don’t always think about context. For something like pregnane-3,20-dione, the answer changes with the dose, the delivery, even the person’s genetics. Each detail reshapes the risk. I’ve heard experts call for stricter safety tests before green-lighting new compound uses. That means long-term follow-up—not just a quick look at short-term symptoms.
The safest path involves transparency from pharmaceutical companies, open access to study data, and feedback from real people who have tried new treatments in controlled settings. Regulators and scientists need to insist on more studies, not fewer. If anyone tries to sell pregnane-3,20-dione as a supplement or therapy without good safety data, consumers deserve a loud warning. No one gains from shortcuts when health is on the line.
I’ve seen plenty of confusion pop up any time someone asks how to handle compounds with long names. Pregnane-3,20-dione fits right into that mess, mostly because people get scared off by the chemistry and overlook everyday steps that keep it safe and stable. If you care about keeping research budgets in check and avoiding ruined batches, storage isn’t a side note. It defines the line between something useful and something useless.
In university labs, I spent a summer tracking the impact of heat on similar steroid compounds. Left by a window, a few vials started breaking down in less than a week—simple sunlight mixed with warmth did the trick. Pregnane-3,20-dione plays by the same rules. Warmth messes with its structure fast. Stick to refrigerators or freezers, whatever you have that stays under 8°C. Even a small bump in temperature can nudge it toward breaking down. This isn’t just theory—watch the color after a few days left out and you’ll see for yourself.
Some people laugh about foil-wrapped bottles, but examine any steroids exposed to regular lab light, and you’ll know why this happens. Pregnane-3,20-dione can start falling apart with enough light, especially ultraviolet. Shut it away in an amber vial or at least keep it in the dark. Simple tricks—black-out tape, dark glass, or wrapping a bottle—extend shelf-life dramatically. I’ve found containers tucked away at the back of a drawer last for years, compared to the stuff on a brightly-lit bench.
Molecules don’t like moisture, and neither does this compound. Dry conditions prevent clumping and slow down all sorts of chemical reactions you’d rather avoid. I once opened a bottle stored near a humidifier and found it had turned sticky, useless for anything serious. Keep silica gel packs inside the boxes. Tighten the cap every time you finish—an open bottle even for an hour can spark trouble, especially if you work where summer heat makes the air feel sticky.
There’s nothing boring about keeping vials uncontaminated. One mistake I’ve seen: using scoops or pipettes from other substances. Pregnane-3,20-dione doesn’t forgive cross-contamination. Even trace bits of another powder or oil throw off results or ruin an experiment outright. Stick with clean tools. Label each bottle down to the batch number, because order saves you from guessing games if a project takes a turn months later.
Getting storage right isn’t about micromanaging every step—just follow some low-tech routines. Keep bottles sealed tight. Use amber glass, steer clear of sunlight, and always handle in a cool room. Invest in a small desiccator box for high-humidity areas; they pay for themselves in preserved material. Encourage teammates to write the open date on the label. It sounds simple, but catching an old bottle before using it heads off disasters.
Attention drifts when the basics seem boring. After enough failed runs and wasted materials, people start respecting the humble plastic bag, the backup fridge, and those annoying silica packets. Pregnane-3,20-dione isn’t rare or magical—you just have to treat it with care. With these habits, the compound holds up and research avoids the silent failures that make lab work such a gamble.
Pregnane-3,20-dione—better recognized as progesterone's older chemical sibling—has a place in labs, pharmaceutical setups, and even the world of chemical synthesis. Step inside any research-focused chemistry lab, chances are you'll find a jar or two, with a white powder nestled inside, labeled as this compound. It’s not just the chemical structure that makes it interesting; it's about what you can actually get your hands on right now if you need some for real-world work.
I remember the first time I handled this compound: it came as a fine powder, packed into a small glass container, looking no different from a kitchen spice. This is the most common way people encounter Pregnane-3,20-dione, but pure crystalline form turns up frequently as well. In the busy world of chemical supply, researchers and companies want something stable and straightforward. Powders and crystals keep well without fuss and allow for easy measuring, whether you need a pinch or a heap.
Some commercial suppliers, especially those working with big pharma, also provide this compound dissolved in solvents—typically ethanol or dimethyl sulfoxide (DMSO). In liquid, Pregnane-3,20-dione becomes ready for dosing into cell cultures and quick biological assays. No need for extra mixing or dissolving, which sounds small but can save time and sanity on a day packed with experiments.
Every scientist and manufacturer watching their workflow knows that the purity of a chemical can make or break results. Pregnane-3,20-dione arrives in various grades—research, pharmaceutical, and industrial—each promising its own certainty about what’s in the bottle.
Laboratories typically ask for 98% or higher purity, and suppliers offer this, sometimes going up to 99% or more for especially picky applications. Pharmaceutical producers push this higher still, with audit trails for removal of contaminants and focus on trace-level testing. No one wants byproducts skewing data or muddying reaction outcomes. In my past research days, a 97% batch once fouled up an experiment, teaching our team the hard way that every missing percent can come back to haunt you.
Lower grades—95% for instance—pop up in industrial settings, particularly when the chemical forms part of a synthesis pathway and slight impurities won’t matter in the final product. It’s cheaper and faster to buy in this form if you don’t need full-on lab-level purity, and that cost saving does add up across big production runs.
One challenge with Pregnane-3,20-dione lies in verifying what purity you actually receive. Not every supplier backs up their claims with full analytical tests like high-performance liquid chromatography (HPLC) or mass spectrometry. The only way around this is to check for up-to-date lab documentation and, if possible, run your own quality analysis at least once on any new supply batch.
Handling can also become a nuisance: the powder easily picks up static, sticking to tools and containers. Reaching for high-purity glassware, anti-static scoops, and always resealing containers keeps things a lot tidier. In liquid form, Pregnane-3,20-dione breaks down faster if exposed to light or heat. Dark bottles, cool storage, and speedy use protect it from changing into something you didn’t order.
The issue of sourcing traces back to regulation and logistics. More transparency across the supply chain—complete with batch-specific analysis—would help everyone from university researchers to industrial buyers. Sharing info about stability, impurities, and storage tips makes life easier and the science better. Pregnane-3,20-dione may sound niche, but the details behind its forms and purity touch the way a lot of labs, students, and workers get their job done right.
| Names | |
| Preferred IUPAC name | pregnane-3,20-dione |
| Other names |
Dihydroprogesterone 10β,17β-Dihydroxy-4-androsten-3-one |
| Pronunciation | /ˈprɛɡ.neɪn θriː ˌtwɛnti daɪˈoʊn/ |
| Identifiers | |
| CAS Number | 120-29-6 |
| Beilstein Reference | 1210784 |
| ChEBI | CHEBI:28722 |
| ChEMBL | CHEMBL1436 |
| ChemSpider | 7272 |
| DrugBank | DB00337 |
| ECHA InfoCard | 100.000.057 |
| EC Number | 1.3.99.4 |
| Gmelin Reference | **114933** |
| KEGG | C00515 |
| MeSH | D011238 |
| PubChem CID | 6241 |
| RTECS number | GF3150000 |
| UNII | FF28EZG64D |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | C007577 |
| Properties | |
| Chemical formula | C21H32O2 |
| Molar mass | 312.45 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Odorless |
| Density | 1.12 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 2.99 |
| Vapor pressure | 5.29E-6 mmHg at 25°C |
| Acidity (pKa) | 12.69 |
| Basicity (pKb) | 3.89 |
| Magnetic susceptibility (χ) | -91.0e-6 cm³/mol |
| Refractive index (nD) | 1.584 |
| Viscosity | 3.16 cP (25 °C) |
| Dipole moment | 3.12 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 395.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −247.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6585 kJ/mol |
| Pharmacology | |
| ATC code | G03DA04 |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P201, P202, P264, P270, P280, P308+P313, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 180°C |
| Autoignition temperature | Autoignition temperature: 470°C |
| Lethal dose or concentration | LD50 (rat, oral): >4000 mg/kg |
| LD50 (median dose) | LD50: 641mg/kg (rat, intraperitoneal) |
| NIOSH | GV2M05I3ZY |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
17α-Hydroxyprogesterone 11-Deoxycorticosterone 21-Deoxycortisone 5α-Dihydroprogesterone Isoprogesterone |