Chemists have been preparing and handling N-Propylamine since the late 19th century, back when basic alkylamines became useful beyond academic curiosities. Early production methods relied on reducing nitropropane with iron or catalytic hydrogenation, a technology that often left plenty to be desired. For years, N-Propylamine largely filled roles in research labs, making appearances in the synthesis of dyes and pharmaceuticals. Over decades, as production methods grew more refined, specialty chemical makers and agricultural suppliers started recognizing its worth, leading to mass manufacturing. In the past, you would only find it in the toolkit of a chemist fiddling with new reactions; today, it is an established part of specialty chemical commerce.
Take a walk through a specialty chemical plant and someone will point out N-Propylamine as a colorless, volatile, flammable liquid amine. Its formula, C3H9N, puts it squarely among the lower alkylamines, but its properties separate it from cousins like methyl or ethylamines. It’s a building block, plain and simple, but a vital one. Whether you open a drum from a European supplier or a barrel from an Asian producer, the content fits the same standards and finds similar downstream uses in pharmaceuticals, pesticides, and corrosion inhibitors.
Anyone storing or moving N-Propylamine will quickly get familiar with its sharp, fishy ammonia-like odor. Its boiling point (48-50°C) makes it easy to distill or lose by accident, while its density sits right under water at about 0.72 g/cm³. Solubility in water and many common solvents is high, letting it slip easily into reaction mixtures or cleaning processes. On the reactivity front, it doesn’t shy away from acids to give salts, or from acyl chlorides to churn out amides. Its vapors can spread fast and ignite with little provocation, so environmental and warehouse safety officers spend a lot of effort keeping it under control.
Regulatory paperwork and transport documents for N-Propylamine always run thick. You’ll find CAS number 107-10-8, UN identification number 1277, a hazard class (flammable, toxic), and careful instructions on storing the material in tightly sealed containers, usually under nitrogen or a non-sparking atmosphere. Purity generally hits above 99%, and specifications track things like color, water content, aliphatic amines, and presence of residual solvents. Anyone moving the drum needs flame-proof labels and incompatible chemical lists at hand. The safety data sheet reads like a checklist for chemical professionals: splash goggles, chemical-resistant gloves, and ventilation are standard because direct skin contact or inhalation will bring trouble.
Industrial production leans mostly on catalytic hydrogenation of propionitrile over nickel or cobalt catalysts. Running the process under moderate pressure and temperature yields a steady stream of N-Propylamine, which then gets purified by fractional distillation. In small labs, people sometimes lean on reduction of n-propyl isonitrile or ammonolysis of 1-chloropropane, but these methods rarely scale efficiently. The large-scale process generates plenty of heat and hydrogen, requiring strict control measures to avoid unplanned reactions or explosions. Companies with decades of experience in amine chemistry have dialed in their process to the point that product quality and consistency rarely turn heads.
N-Propylamine jumps at the chance to form salts with strong acids and amides with carboxylic acid derivatives, making it a favorite for chemists cooking up new molecules. People in research labs use it for alkylation, condensation, and transamination through straightforward protocols. Give it an acyl chloride and you end up with propylamide; react it with formaldehyde and excess formic acid, the Eschweiler–Clarke reaction hands you N,N-dipropylamine. Its lone pair on nitrogen opens up nucleophilic substitutions and quaternization reactions, giving it a special place in synthesizing active ingredients from pharmaceuticals to agrochemicals.
Searching through catalogs, you find N-Propylamine listed under several alternative names: 1-Aminopropane, Monopropylamine, Propan-1-amine, and normal-Propylamine. Each is just a face of the same compound, with naming conventions reflecting quirky standards from IUPAC to commercial shorthand. On shipping manifests in India, it might show up as "Propylamine Technical," whereas German chemical giants might simply mark the drum with "n-Propylamin." The synonyms keep researchers on their toes to avoid mistakes during ordering or handling in multi-lingual supply chains.
Handling N-Propylamine draws on every lesson I learned about chemical safety. Spill response teams keep sand and neutralizing agents near every tank. People count on effective ventilation and leak detection—vapors hang low and ignite in a flash. Direct contact brings irritation; skin exposed to the liquid itches and burns while inhaling the vapor draws coughs and headaches. Storage advice from old-timers sticks: keep the drums out of sun, far from acids or oxidizers, and check vent caps regularly. Industry safety standards dictate approved containers, explosion-proof equipment, and clearly marked exits wherever alkylamines are processed or stored.
N-Propylamine doesn’t make headlines, but it sits at the root of big developments in everyday life. Pharmaceutical producers lean on it for intermediates in antihistamines and blood pressure medicines. Agrochemical labs use it to assemble pesticides and herbicides, especially where selectivity matters. Paint and coating makers depend on its ability to tweak polymer structures for improved durability. It also pops up as a corrosion inhibitor in pipes and machinery, a role that quietly saves millions in replacement costs for factories and refineries. Anyone looking into the synthetic textile industry will see its mark in dye preparation and cross-linking agents. It’s seldom the end product, always the indispensable helper—the strong, invisible link in the supply chain.
Development teams have dedicated plenty of effort to pushing N-Propylamine chemistry further. Medicinal chemists keep adding new groups and swapping out atoms to discover more effective therapies. Polymer and materials researchers harness it for smart coatings and new composites, playing with crosslinking and surface properties. In the past decade, academic papers and patents have focused on greener, safer synthesis, aiming to cut waste and energy use. Some international groups now explore catalytic and biocatalytic processes to fine-tune reaction selectivity and improve yields. Innovation keeps circling back to N-Propylamine because it links up reliably with so many partners, delivers predictable reactions, and always keeps a spot at the research bench.
Workplace safety history teaches a hard lesson with N-Propylamine: short, sharp exposure brings acute symptoms—red eyes, sore throat, headaches, and sometimes confusion. Repeated or heavy exposures stack up with liver and kidney strain, proved out in animal models and injury reports. As an amine, it serves as a skin and respiratory irritant at low concentrations. Reports from industrial incidents consistently stress training, engineering controls, and regular health checks as the best protections. Many countries regulate workplace exposure limits, and recent toxicological studies suggest reproductive and neurological effects at high doses. Factoring these risks, proper handling standards are not negotiable—there’s no shortcut that keeps workers safe outside of robust protocols and real-time monitoring.
The world of specialty chemistry keeps moving, and N-Propylamine stands ready for bigger roles. Sustainable production routes through bio-based feedstocks attract plenty of interest as environmental rules tighten. As pharmaceutical demand rises and agrochemical innovation pushes forward, more efficient catalytic methods will keep value high for producers who can cut costs without sacrificing quality. Better toxicology and risk mitigation may open fresh doors in food contact materials or water treatment technologies. From regulatory reforms to digital lab automation, people in the field expect N-Propylamine to anchor new chemistries for at least another generation—quietly plugging away in the background as essential as ever on the path to safer, smarter, and more sustainable products.
N-Propylamine might sound like a name only chemists toss around, but almost anyone who benefits from modern agriculture or lives in an urban environment meets its effects. I’ve come across N-Propylamine during routine visits to seed treatment plants and whenever I read the fine print on cleaning agents or herbal weed killers. This colorless liquid serves more than just one industry; it quietly shapes a lot of what ends up in our daily lives.
Farmers and crop scientists look to N-Propylamine for its role in making herbicides and fungicides. Many weed killers don’t get their punch without it as a building block. Certain molecules, on their own, won’t cling well to plant tissue or dissolve in spray tanks. N-Propylamine helps tie ingredients together in a way that ensures maximum coverage and effect, so the weed killer sticks to leaves and stays put after rain.
Take glyphosate, a herbicide found in thousands of fields. Farmers rely on it to clear fields before planting, cut costs, and grow more food with less effort. N-Propylamine acts as a key ingredient in producing some glyphosate variants, giving those products the solubility and reliability needed for real-world use. Without it, farms would run less efficiently, and crop yields could drop.
In many factories or plants, greasy floors, tanks, and parts don’t stay clean with water and old-fashioned soap. Complex grime calls for stronger chemistry. N-Propylamine goes into the formulas of heavy-duty detergents and degreasers. Its ability to dissolve tough oils means that engine factories and machine shops snag it for regular cleaning cycles, keeping equipment working and workers safe.
Drug companies find N-Propylamine useful in turning raw ingredients into pills and creams that help real people. Certain painkillers or antihistamines perform better thanks to chemical tweaks involving this amine. In a sense, N-Propylamine often helps the active part of a drug become stable and easy to swallow. If you’ve ever taken a tablet for a cold, you’ve felt its influence.
N-Propylamine works hard in industry, but it’s not something to handle carelessly. It can irritate skin, eyes, or lungs. I've seen safety officers train factory teams to use tight gloves, goggles, and ventilation systems to keep contact limited. Problems rise when rules get skipped or disposal isn’t tight. Wastewater full of N-Propylamine can harm rivers or ground supplies if poured without treatment.
Safe use calls for education and strong workplace rules. Cleaning up spills right away and using sealed transfer systems limit the danger. Waste facilities use advanced filtering and treatment to keep plant runoff in check. Farmers get trained on safe handling and storage, cutting any risk that could roll into streams or fields. Makers and regulators keep sharpening these solutions as new risks pop up.
N-Propylamine may not get the headlines, but it underpins the systems driving modern life and food supply. Its upsides outweigh the hassles, so long as we respect the risks and put strong checks in place. I’ve learned that chemistry, even in small doses, shapes the food on our tables, the pills in our cabinets, and the cleanliness of the machines we depend on. Getting it right means thinking beyond the lab bench—straight into everyday life.
N-Propylamine gets plenty of use in factories and labs, showing up in things like pesticides, rubber, and even pharmaceuticals. You can usually pick it out by its fishy smell and the way it irritates your nose if you get too close. It’s got power, but it packs real risks. My years around the warehouse floor taught me that taking shortcuts with chemicals always backfires. You get away with it, until you don’t—and the day things go wrong, the clean-up can last a lifetime.
Short sleeves and thin gloves aren’t enough. N-Propylamine doesn’t just sting if it gets on your skin; it soaks right in and leaves you with rashes or worse. Chemical-resistant gloves made for organic substances, goggles that seal shut, and a good lab coat block most of the trouble. If the air reeks, that’s a sign—grab a respirator. Paper masks don’t cut it.
I learned early that fans alone can't sweep away dangerous fumes. Fume hoods or strong exhaust systems earn their keep. Poor airflow turns a small spill into an emergency. These fumes irritate your lungs, but over time, the damage builds beneath the surface. I remember the warning of a colleague—after years of ignoring that prickling in his throat, breathing issues never disappeared. That lesson stuck with me.
N-Propylamine, left on a warm shelf or near a heat vent, spells trouble. It ignites easier than gasoline vapors in a hot garage. I once saw a drum swell in the summer heat, and folks scrambled to cool the place down. You keep these containers tightly sealed, tucked in flame-proof cabinets, far from acids or oxidizers. Label everything with clear, bold print. That’s not just policy—it lets you sleep at night knowing you didn’t set a trap for the next guy.
Small drops come with big risk. Absorbent pads, plenty of baking soda solution, and fast cleanup protect both people and ground. Any rags or pads go in special chemical waste bins—never in the regular trash. Training everyone on your crew, from janitor to chemist, means no one fumbles in a crisis. Quick action stops a headache from becoming a hospital trip.
Everyone says “it won’t happen to me” until it does. I keep safety showers and eyewash stations clear, never blocked by boxes or carts. Phone numbers for poison control and emergency services hang right where anyone can find them. A spill kit close to the action, not in a forgotten closet, can make all the difference.
Rules on paper only go so far. Real safety means talking through the “why” behind every step and sharing stories of close calls. In my experience, folks remember lessons when they hear what went wrong for someone else. A culture where people double check each other and speak up without fear changes a careless room into a safe one.
Simple changes go a long way. Adding more visible reminders about glove types, airflow, and storage spots made a world of difference at one site I managed. Refresher courses every six months kept details fresh for everyone, not just the new hires. Safety audits by someone who’s worked with chemicals in the real world—not just read the books—catch problems before they turn ugly. I learned it’s better to sound like a broken record than face the consequences of one missed step.
Chemistry can sometimes feel like a secret language, but a lot of the stuff you encounter each day—cleaners, medicines, even flavors—stem from small, basic molecules like N-propylamine. You might not hear about it often at the dinner table, but factories and laboratories use it because it reacts easily and opens doors to make all sorts of useful things.
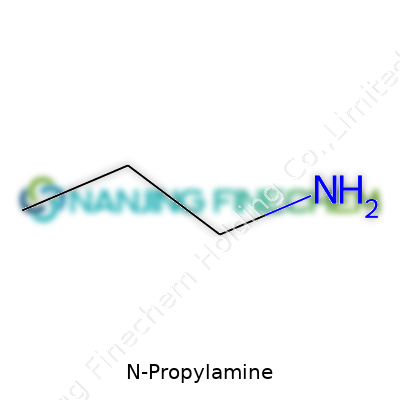
N-propylamine carries the formula C3H9N. Think of this as three carbon atoms, nine hydrogens, and a single nitrogen glued together in a specific order. Kind of like Lego blocks, but the bonds between them set the rules for how it behaves.
The backbone has three carbon atoms—making it a “propyl” chain. Chemists like to name things based on their shape, almost like giving every dog its breed name. Here, “N” stands for the way the ammonia part (NH2) attaches straight onto the first carbon in the chain.
So what does this look like if you sketched it? Imagine three carbon atoms in a row. Stick two hydrogens on each of the end carbons and one on the middle. Attach an amino group (NH2) at the end of this line. Its full structural formula comes out as CH3-CH2-CH2NH2. That’s why you sometimes see N-propylamine called 1-aminopropane.
This amine structure sounds fancy—until you realize it’s just a nitrogen hanging onto two hydrogens, stuck to the very tip of the propyl chain. I remember handling some in college labs. The smell? Rough and fishy, the sort of thing you want to wash off your hands right away. That’s typical with small amines.
The compact shape makes N-propylamine so reactive. Factories count on it as a starting point to build larger molecules. Agrochemical companies and the makers of dyes both tap into this property. I realized working with industrial adhesives that tiny changes to the carbon chain, like swapping out a carbon or shifting that NH2 group, lead to products with entirely new properties—stickiness, flexibility, water resistance.
This isn’t just theory: N-propylamine brings a punch in terms of basicity. Its nitrogen holds a lone pair of electrons, grabbing on to protons that acids offer up. That’s an open invitation for chemical reactions, which is why it does well in labs and factories looking to create more complex materials. And because the propyl group isn’t bulky, other molecules can approach easily. That’s chemistry with elbow room.
Like a lot of these straightforward nitrogen compounds, don’t mess around with it lightly. Breathing it in can really irritate your nose and lungs. Glance at any safety data sheet, and you’ll see it’s handled with gloves and goggles—no exceptions. In my lab experience, the bottle got double-checked before use and always stored in a tightly sealed container in a well-ventilated area.
As industries shift toward greener chemistry, questions come up around sustainable sourcing and safer production. Making and using amines, like N-propylamine, calls for clean processes to keep emissions and spills in check. New research tracks ways to clean up residues or replace solvent-heavy reactions with more environmentally friendly ones.
N-propylamine might look simple on paper, but as you peel back the layers, it shows how much chemistry shapes what we make and use every day. The details—those atoms and bonds—matter more than you’d think.
N-Propylamine isn’t just another bottle on the shelf. It’s a flammable, volatile chemical with a sharp, ammonia-like odor that can wake up a whole room in seconds. At my first job in a small lab, a lapse in storage taught me more than a lecture ever could. Fumes escaped, set off alarms, and scrambled our morning. No one wants to repeat that mess. Simple mistakes can put both health and property on the line.
Sticking N-Propylamine next to strong acids or oxidizers is just asking for trouble. Its formula (C3H9N) might not scream “danger” outright, but mix-ups have turned clear mornings into chaos. In my years working around chemicals, I’ve learned the value of designated areas. Incompatible neighbors spark reactions, so distance them like rowdy classmates in eighth grade.
Keep it away from heat sources, even old radiators or sunny windowsills. This isn’t just about avoiding a fire. Vapors can creep, and if any electrical outlet sparks nearby, that’s all it takes. I’ve watched minor lapses end careers—or worse. An ordinary metal cabinet in the wrong place turns risky fast. Fire-resistant storage cabinets bring peace of mind. You don’t realize how much you appreciate them until the day something goes sideways.
A battered old bottle, unclear label, or loose cap often leads to headaches—sometimes literally. Original containers, solid seals, and clear, heavy-duty labels keep confusion out of the equation. My habit now: double-checking everything before closing up shop. It saves time, mess, and uncertainty. Stainless steel, glass, or high-quality plastic keep the chemical safely contained. Any cheap or rusty container is like storing apples in a paper bag during a rainstorm; it won’t last long and invites disaster.
Open a poorly sealed bottle of N-Propylamine and the smell punches back. Vapors are not only unpleasant—they can also knock out your sense of smell with repeated exposure. Ventilation matters. Storing this stuff in a storage closet with no air movement sets up the entire building for trouble. A well-ventilated area, separate from where people spend hours, keeps everyone healthier and cuts down on surprise evacuations.
Accidents catch everyone off guard. During a spill, people remember fast whether their goggles and gloves sit within reach. Good storage isn’t just about location; it’s about how quickly you can respond when the unexpected happens. Spill kits close at hand have kept my workspace out of headlines more than once. Chemical-resistant gloves, safety goggles, and a fresh lab coat become everyday items, not afterthoughts.
Routine checks work better than last-minute panic. Someone overseeing stockpiles, shelf lives, and container integrity makes a difference. Throwing out expired or cracked containers is cheaper than cleaning up a major spill or dealing with sick employees. Following local fire codes or OSHA guidelines isn’t about bureaucracy; it’s about keeping people and livelihoods safe. Proper storage matches responsibility with real-world consequences—something I learned outside textbooks, the hard way.
Step into a lab, open a bottle of N-Propylamine, and you’ll get hit by a strong, fishy smell that can turn heads. This isn’t some mystery liquid—this is a clear, colorless material with a simple structure that packs a punch. N-Propylamine catches attention for more than just its scent: it’s light, moves easily as a liquid, and evaporates in a hurry. That low boiling point, around 48 degrees Celsius, means it won’t stick around if you leave the cap off too long.
From my days in the organic chemistry lab, I remember N-Propylamine for more than just its sharp odor. Skin feels a slight burn with a splash, and eyes protest if vapors catch you off-guard. This isn’t a chemical to take lightly. It slides through gloves and small leaks with alarming speed because it clings to the lower end of the density chart at roughly 0.74 g/cm³. That means, in plain terms, it’s lighter than water—but a lot more eager to get airborne.
People sometimes think of chemicals as either “mixable” or “not.” N-Propylamine disregards such categories. Pour it into water, alcohol, or ether; it dissolves before you blink. In the real world, this fast mixing means spills can spread wide and fast, making it crucial to work in open spaces or under a fume hood. Its low flash point—about -20 degrees Celsius—makes anything involving flames or static a risky affair. I once saw a small spark near a bottle cap, and the pop could have been a close call if not for plenty of fresh air and quick reflexes.
Handle N-Propylamine like you would handle an overzealous dog: with respect and barriers. It irritates airways, eyes, skin, and signals its presence long before measurements even pick it up. The fact that it forms explosive mixtures with air at room temperature means every cap twist, every transfer, every use needs careful planning. I’ve seen accidents happen because a bottle wasn’t closed tight enough or someone wiped up a spill with the wrong materials—never underestimate how quickly vapors can hover and ignite.
Every lab professional eventually has to find ways to balance usefulness with risk. N-Propylamine has needed substitutions in my projects where large open containers couldn’t be avoided. Ventilation isn’t just a helpful guideline—it’s life-saving. Using the right gloves, goggles, and safe storage, along with training for everyone touching a bottle, saves hassle later. Factories that use this amine in big batches turn to sealed systems and automated transfer lines to pull people away from the danger zone.
N-Propylamine has a reputation built on its physical traits: volatile, flammable, aggressive to the senses. These aren’t just textbook values. Anyone working with it will tell you that each of these properties carries real consequences. Understanding the numbers—density, boiling point, solubility—ends up being about more than passing a test. It’s the difference between chasing health and safety paperwork and going home at the end of the day without a story involving emergency showers. In practical terms, this chemical reminds you every day that safety protocols exist for a reason.
| Names | |
| Preferred IUPAC name | propan-1-amine |
| Other names |
1-Aminopropane 1-Propanamine Propan-1-amine Propylamine |
| Pronunciation | /ɛn ˈproʊ.pəl.əˌmiːn/ |
| Identifiers | |
| CAS Number | 107-10-8 |
| Beilstein Reference | 1700228 |
| ChEBI | CHEBI:16183 |
| ChEMBL | CHEMBL1675 |
| ChemSpider | 5796 |
| DrugBank | DB01941 |
| ECHA InfoCard | 100.003.060 |
| EC Number | 01-2119486297-23-0000 |
| Gmelin Reference | 7782 |
| KEGG | C01574 |
| MeSH | D011474 |
| PubChem CID | 8057 |
| RTECS number | UE9100000 |
| UNII | XDC444472X |
| UN number | UN1277 |
| CompTox Dashboard (EPA) | DTXSID5020660 |
| Properties | |
| Chemical formula | C3H9N |
| Molar mass | 59.11 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Ammonia-like |
| Density | 0.720 g/mL at 25 °C(lit.) |
| Solubility in water | Miscible |
| log P | 0.38 |
| Vapor pressure | 400 mmHg (20 °C) |
| Acidity (pKa) | 10.7 |
| Basicity (pKb) | 3.34 |
| Magnetic susceptibility (χ) | -6.7×10⁻⁹ cm³/mol |
| Refractive index (nD) | 1.385 |
| Viscosity | 0.38 mPa·s (at 20°C) |
| Dipole moment | 1. dipole moment of n-Propylamine: 1.35 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 198.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -20.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3916.6 kJ/mol |
| Pharmacology | |
| ATC code | N01AX10 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H225, H302, H314, H332 |
| Precautionary statements | P210, P261, P280, P301+P310, P305+P351+P338, P331, P403+P233, P501 |
| NFPA 704 (fire diamond) | 3-3-2-A |
| Flash point | *below 0 °F (NTP, 1992)* |
| Autoignition temperature | 398 °C |
| Explosive limits | 2.0% - 10.4% |
| Lethal dose or concentration | LD50 oral rat 820 mg/kg |
| LD50 (median dose) | 730 mg/kg (rat, oral) |
| NIOSH | 46-00 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of N-Propylamine is 10 ppm (parts per million) |
| REL (Recommended) | 30 mg/m³ |
| IDLH (Immediate danger) | 170 ppm |
| Related compounds | |
| Related compounds |
Methylamine Ethylamine Isopropylamine Butylamine Di-n-propylamine Tri-n-propylamine Aniline N,N-Dimethylpropylamine |