People began noticing isobutyronitrile in the early twentieth century. Chemists, always on the lookout for compounds that shape other industries, found that this nitrile came with a short, branched chain. Its production in the lab usually relied on straightforward methods, using available feedstocks. Old textbooks from the 1920s describe researchers adding isobutyronitrile to their toolkit for making new chemicals, especially for intermediates in pharmaceuticals and dyes. Traditional plant setups offered basic purification and distillation methods, and before long, this chemical landed on the supply lists for research labs and specialty manufacturers. The industry only started to keep records of its quantities and distribution when global chemical regulations demanded better labeling and tracking through the latter half of the century.
Isobutyronitrile looks like a clear, colorless liquid—easy to mistake for any number of simple organic solvents on a standard lab shelf unless you glance at the label. It slips into organic synthesis processes and has a reputation for reliability. Its formula—C4H7N—funnels through supply chains headed to flavor, fragrance, and pharmaceutical applications. Companies package it in bulk containers for industrial buyers, and maintain smaller bottles for research and development labs. Manufacturers offer different grades to suit the flux of regulatory and customer pressures. Its relatively low molecular weight makes it easy to transport and store, as long as proper caution is in place.
Isobutyronitrile boils at about 107°C, which means that even a moderate source of heat sends it into vapor. The compound mixes with organic solvents like ether and alcohol, but resists dissolving in water. Its faint, not unpleasant odor carries the telltale sign of many simple nitriles. Flammability presents hazards—vapors can ignite in air at certain concentrations. In a glass bottle, no one notices its presence, but it’s constantly waiting for a reaction if left near a heat source or open flame. Its refractive index lands at 1.378-1.38, and the density hovers around 0.78 g/cm3. The chemical holds a decent level of stability under standard storage but wages a losing battle against strong acids or bases.
Labels for this nitrile need more than a trivial identification. Major suppliers print hazard icons, the UN number (UN 2351), and GHS (Globally Harmonized System) warnings directly on packaging. They also break down purity percentages—99% for most technical applications—as buyers expect a product clear of unknowns. Batch numbers, production date, and expiration round out these stickers so users can track and recall inventory. Technical data sheets show the boiling point, flash point, incompatibilities, and storage limits, all meant to keep workers and property safe when moving and handling this compound.
Most production of isobutyronitrile happens through the amination of isobutyl chloride using sodium cyanide. Every chemist learns that this classic nucleophilic substitution—an SN2 pathway—swaps out the chloride with a cyano group. The transformation carries over to commercial scales, too, where big reactors handle the exothermic nature of the reaction. In smaller research labs, yields often reach over 80% using carefully dried solvents and clean glassware. Some newer approaches skip toxic sodium cyanide in favor of safer chemistry, but old habits die hard in industry-scale production.
Chemists use isobutyronitrile as a jumping-off point for other chemicals. Acid or base hydrolysis unzips the nitrile group to create isobutyric acid—an important intermediate in food and fragrance manufacturing. Grignard reactions welcome the cyanide group as a handle for building more complex molecules. Isobutyronitrile, like many small nitriles, introduces alkyl side chains into heterocycles common to pharmaceuticals. Its modifications can steer synthesis toward alcohols, acids, or amines, depending on the route chosen and the side ingredients tossed in. The simplicity of its structure sometimes hides the value it adds by providing straightforward transformations in multi-step syntheses.
If you search supplier catalogs or chemical registries, isobutyronitrile hides behind names like 2-methylpropanenitrile and isobutyronitrile. Historical literature writes it as isopropyl cyanide or 2-methylpropionitrile. Some companies list it under trade names or catalog numbers, but experienced chemists cross-check molecular structures to avoid confusion. These synonyms reflect a wider tradition in chemistry—shorthand born of convenience or local practice—but everything comes back to C4H7N.
Callous handling or ignorance of isobutyronitrile’s properties leads to avoidable trouble. Inhaling the vapor irritates the eyes and respiratory tract, and the flammability risk brings strict safety protocols. Facilities that store and use this nitrile train workers to use fume hoods, protective gloves, and splash goggles. Storage calls for tightly sealed containers, perched away from any heat source or oxidizer. Spill procedures require knowledge and agility—absorb small leaks with inert materials, and wash spill areas thoroughly. Firefighters treat the compound as a volatile organic hazard. Regulatory agencies classify it as potentially harmful and mark it with codes under REACH, OSHA, and other frameworks.
Isobutyronitrile rarely makes headlines, but the hidden value underpins several key sectors. Pharmaceutical makers craft active molecules and intermediates from its structure. Flavors and fragrances benefit from the branched backbone, since isobutyric derivatives play a role in aroma chemistry. Specialty polymer companies choose nitrile components for certain performance plastics. Laboratory researchers value isobutyronitrile as a test reactant or baseline additive, especially when mapping out multi-step routes. Its routes into finished consumer products rarely get public attention, yet you’ll find its fingerprints in chemicals that color, flavor, and treat goods in daily life.
Recent R&D trends challenge the use of classic cyanation methods—the push for green chemistry asks scientists to design less-toxic synthesis strategies. Enzyme-catalyzed routes and alternative nucleophiles get attention for preparing isobutyronitrile or the derivatives that spring from it. Analysts study the fate of isobutyronitrile in industrial wastewater streams and its potential breakdown into more persistent chemicals. Patent filings show ongoing interest in both direct applications and clever modifications, proving that even a plain-looking nitrile earns steady care from both young scientists and industry veterans searching for cost or performance advantages.
Studies dating back to the mid-twentieth century trace the toxicity profile of isobutyronitrile, especially after a few workplace accidents brought its exposure risks into focus. Acute exposure affects the central nervous system and, at higher doses, brings convulsions, respiratory failure, and—rarely—death. Animal tests outline the dose-response relationship; findings help guide occupational safety limits used by regulatory agencies today. Chronic exposure data points to more subtle risks: skin irritation, headaches, and mild changes in weight or behavior. Toxicologists continue to probe breakdown products and human metabolic pathways, since nitriles sometimes yield toxic compounds once inside the body. The push for improved workplace monitoring and ventilation can’t move forward without this research.
Industry will keep searching for alternatives that avoid cyanide routes—safety, cost, and environmental pressure make that path unavoidable. Bio-based raw materials may open new windows for making isobutyronitrile, with researchers working out ways to scale up enzyme reactions or microbial fermentations. Flavors, pharmaceuticals, and materials science all have room for more sustainable intermediates, but change moves slowly, especially in big plants with legacy infrastructure. Regulations keep tightening, so companies juggle compliance with efficiency. Chemists on the ground know that the right molecule today must still answer tomorrow’s safety and environmental questions, shaping how isobutyronitrile moves from factory floor to product shelf.
Isobutyronitrile doesn't show up on grocery lists or in home improvement aisles, but it plays a quiet role in things we rely on every day. Digging into my years working with chemical supply businesses, I’ve seen the demand pop up in some unexpected places. That clear liquid, also known as isopropyl cyanide, ties into industrial routines and, more interestingly, into our modern routines through products we don’t stop to notice.
Pharmaceutical research hinges on reliability and precision, and that’s where isobutyronitrile gets its ticket punched. Scientists use it as a building block for specialty drugs. Its structure allows tweaks and changes at a molecular level, making it valuable for synthesizing new medicine candidates. I remember researchers swearing by the compound’s consistency during pilot trials for new drugs. It’s not front and center, but without it, the show often can't go on.
Farmers don’t spread isobutyronitrile over fields. Still, back at the plant, agricultural chemical manufacturers depend on it to shape active ingredients. Pesticides, fungicides, and some weed killers begin their journey with this chemical. These aren’t just big words—these are the products that keep tomatoes safe from fungus and corn from weeds. I’ve watched batches come together in plants where one misplaced substitution could ruin thousands of gallons of chemical solution, but isobutyronitrile keeps showing up for the task.
Industry loves durable plastics and effective resins. As I followed the supply chain for plastics, I learned that isobutyronitrile often forms one of the first steps for certain acrylics. These acrylic resins aren’t just the stuff in plexiglass—they’re used in paints that last longer, adhesives that don’t give up, and specialty coatings for electronics. Throughout manufacturing, even a small miscalculation in purity can scramble an entire lot, so keeping isobutyronitrile pure and available has always been a top concern for buyers.
Now, not everything with ‘nitrile’ in the name is gentle on the nose or skin. Safety folks on plant floors know how finicky—and sometimes hazardous—these chemicals can be. Handling isobutyronitrile without proper gear can lead to headaches, irritation, or worse. Early in my career, I watched a crew train new hands on storage and breathing safety, drilling the lesson that shortcuts never pay off. Better ventilation and more robust detection systems could still help, as could stronger regulations on waste disposal.
Waste streams and runoff have made headlines far from industrial parks. I’ve seen companies work harder on recycling steps, converting leftover isobutyronitrile into less worrisome substances before shipping barrels off for disposal. Newer processes also aim to use less solvent and energy, catching resource-conscious eyes in places hit hardest by pollution. Investing in these improvements isn’t just ethical—it’s practical for communities downstream from production.
For folks outside of labs and factories, isobutyronitrile might look like just another entry on a long list of chemicals. Yet, it’s clear that its uses shape everyday life, whether it’s drug discovery, sturdier paints, or keeping food on the table. Each small change toward safer use and cleaner production shapes the future—one batch, one project, one day at a time.
Dealing with chemicals like isobutyronitrile isn't just for folks in white coats. Anyone who spends time in a lab or works around manufacturing knows this substance deserves respect. It’s colorless and looks pretty harmless on the surface, but don’t let that fool you. The stuff is flammable, and the fumes can sting your nose, eyes, and throat before you even realize what’s going on. That’s why preparation isn’t just routine—it’s common sense.
One of the biggest mistakes people make with chemicals is banking on luck or convincing themselves that a quick task doesn’t call for full gear. Gloves and goggles aren’t for show. I’ve learned from a friend working in plastics that nitrile gloves do the trick for skin protection, and if he ever forgot his eye protection, he’d regret it by the end of the shift. Cover your eyes, pull on a chemical-resistant apron, and make sure closed-toe shoes are on before you even open the container.
Ventilation matters a lot more than most people think. Even a faint smell tells you that volatile stuff is escaping. Breathing in isobutyronitrile vapors can irritate your lungs, and it isn’t something you shake off easily. Mechanical ventilation or fume hoods can pull fumes away fast. Don’t underestimate how quickly a headache can set in from poor airflow. In smaller workspaces, portable extraction fans or even just opening a window can help, but enclosed, stuffy rooms create danger fast.
Fire isn’t a far-fetched risk here. I spoke with a technician who once worked with nitriles, and he kept all sources of sparks far from his chemical bench. That means no smoking, no hot plates, no loose wires. Use non-sparking tools, and check that all your electrical outlets are up to code. Store your isobutyronitrile in flame-resistant cabinets; don’t just leave it on any shelf because those vapors look for trouble if they leak.
A little spill can happen to anyone. Absorb it with proper materials, not just the nearest rag or paper towel. Specialized absorbents soak up dangerous chemicals safely. Don’t try to sweep anything under the rug. If you get the liquid on your skin, rinse for at least 15 minutes, not just a quick wash. If fumes start making you dizzy, step outside right away—don’t try to tough it out.
Finishing up isn’t the finish line if waste sits around. Mix isobutyronitrile in with the wrong trash and you’re asking for fires or toxic leaks. Always use containers made for hazardous chemicals, clearly marked, and send them off with certified handlers. Local rules lay down the law on disposal, so it pays to check what your area expects. Cutting corners just lands you or someone else in trouble down the road.
Fatigue or distractions can trip up even careful workers. Take breaks, work with a buddy if you’re dealing with big batches, and keep emergency contact info right next to the doors. A safety shower and eyewash station nearby can mean the difference between a close call and a serious injury. It’s not paranoia—it’s experience speaking. Take these steps, and you keep both your lab and yourself in one piece.
Isobutyronitrile sounds like a mouthful, but its structure isn’t too tough to picture. The core of this molecule features four carbon atoms. Three of them bunch together in a branching pattern, and the fourth one extends out, tightly bonded to a nitrogen, making up the nitrile group. So, structurally, you’re looking at something that scientists often write as 2-methylpropanenitrile, or by chemical shorthand, isobutyronitrile (C₄H₇N).
At the heart of its structure, you get a central carbon that branches off in two directions—two methyl groups (–CH₃) on one side, a hydrogen sticking out, and then the cyanide part (–C≡N) attached. The shape matters. The branching of carbons changes how the molecule reacts in chemical processes, compared to a straight-chain cousin like butyronitrile.
Anyone who has spent time mixing chemicals in a lab knows the structure is more than just dots and lines on paper. It dictates flammability, toxicity, solubility, and even smell—the building blocks of how stuff behaves in the real world. Isobutyronitrile has a structure that makes it easier to spot by scent, for example, as nitrile compounds can pack a pungent, sharp odor that clings to memory.
This kind of molecule pops up beyond the chemistry classroom. The nitrile group draws heavy use in industry because it’s reactive. Chemists rely on nitrile-containing molecules like isobutyronitrile when making pharmaceuticals or specialized chemical intermediates. In my own undergraduate days, handling nitriles meant careful attention, gloves, and plenty of ventilation—they do not sit benignly on a shelf.
One of the more interesting facts: isobutyronitrile has shown up outside Earth, detected in interstellar space. This opens up questions about the role of branching molecules in the formation of complex organic compounds far beyond our planet. Space scientists study such molecules while hunting for clues about how life’s building blocks might form in the cold dark, far from a lab or factory.
Another angle comes from process safety. The nitrile group is potent. Without the right precautions, it becomes a health hazard—exposure risks include headaches, dizziness, or much worse in high enough doses. Regular folks won’t see isobutyronitrile in daily life, but its structure teaches a lesson: small changes, like branching one carbon, shift properties in ways that matter. I remember an incident during a summer research project, where misreading a molecular formula led to using isobutyronitrile instead of its straight-chain cousin. Cleanup was no picnic—branching raised volatility, spreading the strong smell quickly and requiring more rigorous disposal steps. That mistake hammered home how much structure actually matters.
Looking to solutions—making chemical processes safer often hinges on understanding molecules like isobutyronitrile from the ground up. If you can swap out a more hazardous substance for one with a similar effect but less risk, everyone wins. Training lab workers, students, and technicians to actually picture the shapes and branches, not just memorize names, goes a long way toward building a safer, more efficient workplace. Chemical structure isn’t abstract; it shapes the choices and safety of anyone who works with these compounds.
Isobutyronitrile might not show up in most people’s conversations, but it moves through some corners of the chemical industry. Anyone working in labs, manufacturing, or supply chains for fine chemicals can come across it. Every time I’ve walked into a chemical storeroom, I pay attention to what’s around, and stuff like isobutyronitrile makes me keep my guard up. It has a reputation for toxicity, and that’s not just a rumor floating through breakrooms.
Labels on isobutyronitrile containers don’t exaggerate. The liquid itself gives off toxic fumes if you let it heat up or spill, and the smell, while not the strongest, offers a harsh reminder that your lungs and skin don’t like it. According to safety documents, this compound can cause serious harm through inhalation, skin absorption, or ingestion. In scientific settings, even a splash on the skin can set off redness, or worse, systemic toxicity leading to headaches or nausea.
One big risk draws from its chemical makeup. Isobutyronitrile counts as a nitrile, and that’s a class known for releasing cyanide when metabolized by the body. It’s scary to know that if mishandled, accidental poisoning isn’t out of the question. Data from the National Institute for Occupational Safety and Health (NIOSH) flags this as a chemical with both acute and chronic health hazards. Lab workers have found that direct exposure leaves them with burning sensations on the skin, and inhaling it can cause respiratory problems or even loss of consciousness in high enough doses.
Most folks outside specialty industries aren’t going to bump into isobutyronitrile. Its main role lands in chemical synthesis, not stores or home cleaning products. Still, the professionals handling it can’t cut corners. From sight, it looks plain, almost harmless—a clear liquid. This deceptive appearance makes solid procedures even more necessary.
Industries working with isobutyronitrile usually enforce strict engineering controls. Think: closed systems, chemical fume hoods, routine air monitoring, pre-job safety briefs, full PPE. No one wants to gamble with this stuff. A workplace I visited had a strict "buddy system" for handling nitriles. Staff underwent frequent training—not just videos, but real drills—because knowing how to respond after a splash or spill can spell the difference between a near-miss and a trip to the ER.
Good chemistry doesn’t happen without good safety. Safety means more than gloves; it means planning. Spill kits, eyewash stations, and clear communication between workers keep exposure risks much lower. Simple fixes like keeping containers tightly sealed or working with small quantities at a time make a massive difference. I’ve seen places invest in advanced ventilation for even small-scale batches, just to prevent any vapor build-up.
Clear labeling and easy-to-read safety data sheets keep everyone on the same page. Regular health monitoring for those handling nitriles catches problems early, before symptoms mess with someone’s life or career. Substitution, too—using less toxic chemicals whenever possible—slices away at overall risk.
There’s always pressure on manufacturers and labs to step up safety, especially with substances like isobutyronitrile. No one wants to become tomorrow’s accident statistic. Building a safety culture, speaking up when something seems off, and keeping up with evolving best practices all help shield people from the nastier side of chemical science. The best solution isn’t just the right gear or the right ventilation; it’s a mindset that values every worker’s health as much as any finished product.
Ask anyone who’s worked in a chemistry lab or industrial plant—chemicals like isobutyronitrile have a way of reminding us that shortcuts come with a cost. It’s part of life that someone, somewhere, skips the safety data sheet and pays for it later. Isobutyronitrile poses real risks: it can release toxic fumes, catches fire easily, and has a nasty track record for causing headaches, dizziness, and worse if inhaled. Over the years, neglected flasks and poorly sealed drums have sparked evacuations and sent folks running to eyewash stations. Stories like these show the difference clear protocols make.
Stick this stuff in the right spot, and you nip half the problems in the bud. Tanks or containers built from sturdy steel or tough plastics hold up well to the chemical. Glass works only if you stash it far from places where it can crash, and keep it tight with chemical-resistant caps. Temperature swings spell danger. Keep isobutyronitrile below room temperature—a plain old fridge, reserved for chemicals, gets the job done in smaller labs. In big facilities, walk-in storage rooms with proper venting hold the line against vapors building up.
Nothing beats a good label. Permanent marker, big and clear, right on the bottle—nobody wants confusion when they reach for a reagent in a rush. You don’t just toss these bottles onto any shelf, either. Store isobutyronitrile away from oxidizers and acids—combining the wrong chemicals kicks off reactions faster than you can say “cleanup crew.”
Anyone who’s opened a container of isobutyronitrile knows the smell hits you. It’s the sort that makes your eyes sting if you lean in too close. So, don’t lean. Goggles, gloves, and a lab coat aren’t just for show. Ventilation means running a fume hood, or at least cracking a window if you’re stuck for options—anything to keep the fresh air moving.
I remember a day some years back: a spilled vial and a delayed reaction. We scrambled for clean-up supplies and paid for working too fast. You learn fast that with chemicals like this, rushing keeps no one safe. Regular safety drills and knowing where the nearest eyewash station sits saves time—and sometimes more than that.
Pouring leftovers down the drain lands cities in trouble. It travels, mixes with things you didn’t plan on, and makes for a nasty mess in the water supply. Real disposal happens through official, licensed waste handlers. Call them, get the right containers—usually sealed steel drums—and stop thinking the job’s done until they pick it up. Small-scale setups often rely on hazardous waste collection days. Don’t fudge the paperwork; tracking what goes out matters when audits roll around or something gets traced back.
Some filters or absorbent blends can help mop up small spills before wrapping them up for hazardous waste pickup, but tossing anything chemically soaked in household trash just shifts the problem into someone else’s backyard. Extra effort now saves pain and payments later.
No single company or lab can solve everything, but sharing know-how helps everyone dodge accidents. Seasonal safety refresher courses, clear signage, and taking time to run a double-check on containers pay off. Laws change, but the risks stick around. Following up with local regulations keeps everyone on the right side of inspections and keeps the environment cleaner for the next team.
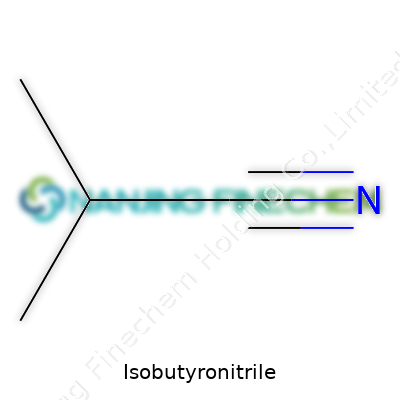
| Names | |
| Preferred IUPAC name | 2-Methylpropanenitrile |
| Other names |
2-Methylpropanenitrile Isopropyl cyanide Isobutyric acid nitrile |
| Pronunciation | /ˌaɪ.soʊ.bjuːˈtɪr.oʊˌnaɪ.trɪl/ |
| Identifiers | |
| CAS Number | 78-82-0 |
| 3D model (JSmol) | `Isobutyronitrile|JSmol|C(C(C)C)#N` |
| Beilstein Reference | 1209249 |
| ChEBI | CHEBI:28271 |
| ChEMBL | CHEMBL16260 |
| ChemSpider | 10443 |
| DrugBank | DB04230 |
| ECHA InfoCard | 100.005.399 |
| EC Number | 607-110-00-7 |
| Gmelin Reference | 877 |
| KEGG | C01781 |
| MeSH | D008409 |
| PubChem CID | 6577 |
| RTECS number | UF3325000 |
| UNII | D7J9XANO89 |
| UN number | UN2481 |
| CompTox Dashboard (EPA) | Isobutyronitrile CompTox Dashboard (EPA) identifier: **DTXSID9020241** |
| Properties | |
| Chemical formula | C4H7N |
| Molar mass | 69.11 g/mol |
| Appearance | Colorless liquid |
| Odor | Faintly aromatic |
| Density | 0.793 g/mL at 25 °C |
| Solubility in water | 5.5 g/L (20 °C) |
| log P | 0.97 |
| Vapor pressure | 2.2 kPa (at 20 °C) |
| Acidity (pKa) | pKa = 25.0 |
| Basicity (pKb) | 4.77 |
| Magnetic susceptibility (χ) | -41.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.342 |
| Viscosity | 0.424 mPa·s (25 °C) |
| Dipole moment | 3.80 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S°298 = 308.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -65.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2226 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Danger |
| Hazard statements | H226, H302, H332, H412 |
| Precautionary statements | P210, P261, P280, P301+P312, P304+P340, P312, P403+P233 |
| NFPA 704 (fire diamond) | 3-3-2-🔥 |
| Flash point | 6 °C |
| Autoignition temperature | 644°C |
| Explosive limits | 1.8–10.6% |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Isobutyronitrile: 640 mg/kg (rat, oral) |
| NIOSH | SN2975000 |
| PEL (Permissible) | PEL = 5 ppm (18 mg/m3) |
| REL (Recommended) | 10 ppm |
| IDLH (Immediate danger) | IDHL: 200 ppm |
| Related compounds | |
| Related compounds |
Acetonitrile Propionitrile n-Butyronitrile Methacrylonitrile Isovaleronitrile |