4-Pregnene-3,20-dione isn’t a name you’ll spot at the grocery store, but its influence runs far beyond any pharmacy shelf. In the 1930s, researchers discovered this compound in the pursuit of understanding hormones that shape everything from stress to reproduction. Back then, the world was still learning how hormones regulate the body. 4-Pregnene-3,20-dione, better known as progesterone, became central in the development of medications that now help millions. Early chemical extractions from animal sources gave way to plant-based and later, synthetic methods. By the middle of the last century, chemists figured out how to synthesize it from diosgenin, isolated from Mexican yams. These advances triggered a wave of medical uses, particularly in women’s health, everywhere from birth control pills to hormone therapy. Today, research into this compound still pops up in journals as experts dive into everything from cancer treatment to neurobiology.
Market shelves and laboratory catalogs show up with 4-Pregnene-3,20-dione under many guises, but at heart, this molecule acts as a steroid hormone. In medicine, its primary use orbits around hormone replacement therapies, managing miscarriage risk, menstrual disorders, and even some types of cancer. Pharmaceutical companies rely on it as a hormone and a stepping stone for producing other steroids. The agricultural sector taps into it through livestock breeding management, improving reproductive performance. Each industry leans on unique formulations — from raw powders to solutions — based on what their users demand.
Breaking down its fine structure, 4-Pregnene-3,20-dione comes as a white to creamy crystalline powder. Melting points hover around 126 to 131 °C. It doesn’t dissolve in water all that well, but organic solvents like chloroform and ethanol do the trick. Its molecular formula sits at C21H30O2, with a molecular weight of about 314.47 g/mol. This structure means any lab technician blending a formulation needs to handle it with a steady hand — moisture, light, and temperature can tip the balance, turning an effective drug into a useless one.
Open a pharmaceutical dossier and the paperwork lays out standards for purity, specific rotation, and trace solvent residues. There’s nothing casual about handling chemicals that affect human health. Labels on 4-Pregnene-3,20-dione products always list the purity levels, often topping 99%, along with lot numbers, dates, and safety instructions. Labels and certificates track each batch in a chain of custody from factory to pharmacy, keeping risk of error or contamination at bay. GMP compliance marks every step, laying out unambiguous requirements for sourcing, manufacturing, and recordkeeping.
Decades ago, the world relied on animal extractions, tearing down animal glands for tiny amounts of this hormone. Factories now process plant steroids, mostly diosgenin, through a cascade of chemical steps called marker degradation and oxidation, churning out large, reproducible quantities of 4-Pregnene-3,20-dione. Industrial players favor this scalable process over unpredictable animal approaches. Each phase — from solvent extraction, purification, drying, and crystallization — runs under careful watch to stick to quality standards. Chemical engineers aim for efficiency, squeezing more product from less raw material wherever technology allows.
Chemists rarely stop with one compound. 4-Pregnene-3,20-dione’s structure acts like a backbone for building all sorts of therapeutic molecules. Developers convert it into corticosteroids, anabolic steroids, and other progestogens through reactions including hydrogenation, hydroxylation, and esterification. Each tweak changes how the molecule acts inside the body. In pharmaceutical synthesis, slight modifications change half-life, potency, and even what diseases the new molecule can address. That flexibility keeps it central to drug discovery and, by extension, ongoing medical progress.
Scientists and doctors throw around names like progesterone, luteal hormone, and corpus luteum hormone for 4-Pregnene-3,20-dione. Chemical catalogs list synonyms including 17α-hydroxyprogesterone and Pregn-4-ene-3,20-dione. Pharmacies carry it under commercial names, each tailored for branding in different global markets. No matter the label, professionals know the molecule and its implications before prescribing or handling.
Safety starts with knowledge. This compound requires careful handling, from production to disposal, because skin contact and inhalation risk serious side effects. Production workers suit up with gloves, goggles, and respirators, while ventilation systems pull trace hormone dust out of the air. Storage in tightly sealed, amber-tinted containers keeps light and moisture away, maintaining stability. Pharmaceutical guidelines, such as USP and EMA monographs, set limits for impurities and mandate regular testing at set intervals. Emergency protocols address accidental exposure right alongside more routine quality checks, reflecting hard lessons from years in the industry.
Doctors lean on 4-Pregnene-3,20-dione for pregnancy support, hormone therapy during menopause, and treating gynecological disorders. The fertility industry counts on it for embryo implantation and ongoing pregnancy maintenance. Cancer researchers test synthetic versions as adjuncts in treating endometrial, breast, and prostate cancers. Beyond medicine, some livestock breeders use it for cycle control or to synchronize animal reproduction in herd management. Researchers designing new drug molecules often start with its structure. Its impact reaches into every corner of hormonal healthcare, with a new study appearing every few weeks to shine light on fresh possibilities.
Big pharma and academic teams keep the pace up on R&D. Molecular tweaking continues, mostly targeting longer-acting or more selective derivatives. Labs jockey to solve delivery challenges: oral, transdermal, vaginal, you name it. Bioidentical forms see intensive testing in hopes of reducing side effects and boosting results for conditions such as preterm birth or hormone-sensitive cancers. Analysts continue to compare natural to synthetic sources, looking for better yield, lower cost, and improved sustainability. Green chemistry initiatives enter the conversation, seeking cleaner production that leaves less waste and consumes fewer resources. The competition keeps prices moving and opens new options for patients tired of old, clunky therapies.
No powerful drug skates past toxicology screens. 4-Pregnene-3,20-dione has shown, in various rodent and primate tests, that high doses over long stretches can toss reproductive cycles off track, spark blood clots, or in rare cases, push up cancer risks in sensitive tissues. Human studies focus on dosing balance, seeking enough benefit with the lowest possible risk. Patients with clotting disorders, liver trouble, or hormone-sensitive tumors always get extra scrutiny. Drug developers and regulators stick to strict review protocols, updating guidelines at any sign of new risks. Education around adverse effects builds into every therapy plan, with researchers chasing improved derivatives for even safer use.
Development won’t slow anytime soon. Drug delivery technologies open new doors: slow-release patches, biodegradable implants, and even nano-carriers that target therapy right where the patient needs it. As reproductive medicine keeps evolving, so does the hunt for better, more tolerable hormone treatments that suit everything from aging populations to rare disease management. Gene editing and tissue engineering raise the prospect of even more selective hormone action, though ethical and regulatory challenges loom. Alongside new applications, sustainability efforts in synthesis and production will get more attention, especially as raw material sourcing and waste generation face tighter regulatory scrutiny worldwide. Each new breakthrough stands on the shoulders of years of hard-earned knowledge about this old, but never tired, molecular workhorse.
Most people never hear the name 4-Pregnene-3,20-dione outside a lab. Countless folks have seen products made from it, though. This molecule, better known as progesterone, shows up on ingredient lists for medicines mostly tied to women’s health. I remember learning about it during my biology undergrad, and even then it sounded too technical or distant to touch real lives. Time proved otherwise.
This isn’t just chemistry for the sake of it. Progesterone sits right in the middle of real stories—families, pregnancies, and much-needed medicines. A doctor prescribes it when a patient’s own body stops making enough. For women who can’t carry a pregnancy because their hormones run low, a capsule or gel form of this molecule can mean the difference between hope and heartbreak. A lot of women struggling with irregular periods, menopause symptoms, or endometriosis rely on it as part of hormone therapy.
Progesterone’s reach stretches past just reproductive health. I’ve sat through talks where doctors discussed its wider effects, from helping reset sleep for people with certain disorders, to easing anxiety by dampening stress circuits in the brain. Some progestins, made using that same backbone structure as 4-Pregnene-3,20-dione, end up as ingredients in birth control pills and hormone replacement therapy. Yet, its main job stays clear—balancing and supporting the reproductive system.
Pharmaceutical companies crave consistency and predictability. 4-Pregnene-3,20-dione gives them exactly what they need to build vital drugs. They start with this molecule and convert it into dozens of other useful compounds. Corticosteroids help reduce swelling, asthma attacks, and skin inflammation because chemists knew how to manipulate this structure. Even if names change by the time it hits a pharmacy shelf, it all ties back to the simple skeleton of 4-Pregnene-3,20-dione.
In the past, scientists extracted progesterone from animal tissue. Later, they switched to plant sources like Mexican yams. Today, most large-scale production relies on semi-synthesis—a mix of plant molecules and chemical ingenuity. This improves access, but prices and supply still fluctuate. I’ve seen patients struggle to afford hormone therapy even when science has all the answers. That gap highlights trouble with health insurance, supply chain hiccups, and how drug pricing doesn’t always serve the people most in need.
More affordable, reliable access to these hormone therapies matters. Patient advocacy pushes for better insurance coverage and transparency in pharmaceutical pricing. Companies have started pilot programs to cut costs for low-income patients. Academic research searches for cheaper, greener ways to create progesterone from plentiful plant sources or even microbes. Expanded telemedicine can help rural patients get these medicines without traveling far from home.
Understanding the use and value of this molecule brings us back to basics—solving problems with science, making sure discoveries turn into better, real-world care. That connection, between science and day-to-day life, keeps me hopeful about where we go from here.
The name 4-Pregnene-3,20-dione doesn’t roll off the tongue, but you’ve probably heard it called something else: progesterone. For a compound with such a huge role in medicine and human biology, its chemical backbone deserves more attention outside academic circles. Looking at its structure unravels much more than a tangle of carbon and hydrogen—there’s history, health, and even agriculture running through these bonds.
Every steroid starts with the same raw structure—a system known as the cyclopentanoperhydrophenanthrene ring. Imagine a pile-up of three hexagonal rings and a squashed pentagon. You’ll find this backbone in everything from cholesterol to cortisol. The structure is rigid but loaded with possibilities. If you look at 4-Pregnene-3,20-dione, you’ll see the core steroid ring system with a handful of tweaks that set it apart. This deserves attention, since subtle changes shape the difference between a hormone, a vitamin, or a poison.
At carbon number 4, you get a double bond—that’s what the “4-ene” part means. Then, you spot two important groups: carbonyls sitting at positions 3 and 20, which together mark the “3,20-dione” portion of the name. Chemists like using shorthand. Instead of a fancy name, they write the molecular formula as C21H28O2. The shape gives the molecule a certain stiffness and polarity, letting it slip into special hormone receptors and influence gene expression.
Ask anyone who’s relied on hormone therapy or oral contraceptives, and the importance becomes obvious. Progesterone isn’t just floating around in plants and animals for fun. That double bond and pair of carbonyl groups let it dock in specific places in the body. Even minor tweaks to the backbone can shift how a medicine interacts inside us, or whether it survives the gauntlet of human metabolism. The structure of progesterone shows why we can’t treat all steroids as interchangeable—each tweak in the architecture brings its own consequences.
I remember my own time poring over steroid structures in college, and nothing forced me to appreciate tiny details like a botched synthetic route. The wrong group on the wrong carbon and suddenly you’ve made something with a totally different biological impact. Drug design lives and dies by the details of a structure like this. Antibiotic resistance, cancer treatments, fertility management—understanding basic chemical frameworks unlocks real-world advances.
Researchers looking to tweak effects or reduce side effects rely on deep knowledge of the “skeleton” and its decorations. Sometimes a small adjustment—moving a double bond, swapping a methyl group—lets scientists dial up benefits or reduce risks. Even in food, traces of this molecule guide livestock growth and reproductive cycles. Spotting these footprints in the molecular detail helps balance innovation with safety, especially as new synthetic pathways pop up faster than ever.
If science classrooms put more effort into stories behind structures, maybe more people would understand how chemical architecture impacts health and society. The double bonds, carbonyls, and rings in 4-Pregnene-3,20-dione continue to drive discoveries that ripple far outside the lab, shaping medicine, agriculture, and beyond. Each atom in this skeleton ties back to real choices—whether that means treating disease or safeguarding how we use hormones in daily life.
Few things scramble the brain more than chemical names. Phrases like “4-Pregnene-3,20-Dione” sound more at home in a high school chemistry test than in day-to-day conversation. This tongue-twister actually points to something a lot more familiar. It’s the chemical name for progesterone, the hormone that quietly shapes so many cycles and stages in life.
Chemists love systematic names. They help tell us what’s in the bottle and how the atoms stack up. In everyday language, though, nobody asks their pharmacist for “4-Pregnene-3,20-Dione.” We call it by its snappier, user-friendly name: progesterone. Both names land on the same compound—carbon, hydrogen, and oxygen atoms forming the same shape, nudging the same biological levers.
Anyone who’s spent time navigating birth control options, fertility treatments, or hormone therapy bumps into progesterone pretty quickly. In the human body, this hormone helps ready the uterus for pregnancy, balances out estrogen, and keeps monthly cycles in line. Medical professionals often prescribe it in pills, creams, and shots.
I’ve seen progesterone’s reputation ride several waves—admired for its key role in pregnancy, eyed warily for side effects in synthetic forms, and even tagged in recent studies for possible links to cancer risk or mood swings. People want the straight story, especially since progesterone supplements can make a huge difference in quality of life for some folks, and for others, raise tough questions about long-term health.
Labels confuse more than clarify in drugstore aisles. Some products say “natural progesterone,” others lean on science jargon. The term “bioidentical” gets tossed around, often without clear explanation. In plain terms, both “progesterone” and “4-Pregnene-3,20-Dione” describe the form found in human bodies. Synthetic versions, like medroxyprogesterone acetate, take a sidestep in their chemical structure (and sometimes, in their effects as well).
This difference matters. Molecular tweaks on the original structure change how these hormones interact in the body. Bioidentical forms, with a matching setup to human progesterone, plug into the body’s receptors with predictability. Altered forms can bring more unpredictable side effects or benefits.
I've chatted with friends who spent years wading through insurance plans and price tags just to get the hormone treatments that best fit their bodies. Some had to argue their way to a prescription, others paid out-of-pocket for compounded versions that used “4-Pregnene-3,20-Dione” (progesterone) but labeled it differently. The U.S. Food and Drug Administration sees these products as interchangeable in most cases, but folks still wind up confused at the pharmacy counter because of shifting labels and names.
Health education, clearer drug labeling, and honest conversations with healthcare providers can untangle this web. Nobody needs another obstacle just because of naming games. With all the changes, stresses, and choices modern life throws at people, knowing exactly what you’re putting in your body, and why, shouldn’t take a chemistry degree.
4-Pregnene-3,20-dione, also known as progesterone, matters a lot in pharmaceutical labs and chemical storage rooms. Anyone who’s spent time around hormone powders knows these aren’t just boring white substances; they’re potent and sensitive. Keeping their structure intact is crucial. I remember working with hormone samples in school, seeing degradation wreck weeks of work just because someone left a reagent cabinet ajar for a few hot days.
Let’s call it straight: heat, light, and water vapor are the enemies here. Progesterone powders look stable, but heat can speed up chemical breakdown. Leave the container in a warm lab or forget it in a sunlit window and you’re looking at impurities in a matter of days or weeks. What’s worse, it becomes less effective for real research or clinical purposes.
Light can do its own quiet damage. Fluorescent lamps and sunlight set up a slow-motion disaster by sparking off degradation reactions, often before anyone can spot trouble. Moisture’s just as sly. Even with tight lids, humidity drifts in over time and can turn a dry powder clumpy. Some friends in small labs try to get around this by keeping desiccants in their chemical closets. Still, there’s nothing like an actual dry cabinet or a dedicated refrigerator for the job.
Science storage advice often boils down to bland phrases like “cool and dry.” In real labs, that means a steady 2–8°C in an actual lab fridge, with no freeze-thaw cycles. I’ve seen people try kitchen fridges, but those swing in temperature every time someone gets a soda. That’s bad news for hormone compounds. Stability takes a hit with those brief temperature spikes and dips.
A pharmaceutical refrigerator offers a more stable environment, and a backup generator keeps things running in power outages. I once worked in a building famous for summer blackouts, and after two within a week, thousands of dollars of research-grade powders had to be trashed. Refrigerators with built-in alarms and data loggers catch problems early, sparing a lot of time and budget.
Most 4-Pregnene-3,20-dione arrives in amber glass bottles or foil-wrapped pouches. That’s not random. Amber glass blocks most UV rays, and airtight seals keep out moisture. Double-bagging in polyethylene or mylar is a cheap trick that works surprisingly well. Squeeze out extra air; drop in a silica gel packet. It’s not overkill—it’s insurance.
A sharp permanent marker and clear labeling top off good practice. Write the opening date, lot number, and storage conditions right on the bottle. In busy labs, people forget what’s in unlabeled vials. A simple date can mean the difference between safe, potent material and junk that throws off sensitive experiments.
Handling and storage go hand in hand. I’ve met many folks who know chemical formulas but ignore best practices once the doors shut. One person’s shortcut can spoil a batch for everyone. Checking for color changes, caking, or weird smells from time to time saves everyone headaches later. People who use the compound should know why these steps matter.
Taking a little care keeps the science—and the budget—on track. Safe storage for 4-Pregnene-3,20-dione pays off every day it stays stable and ready to do its job.
Many chemists and lab techs recognize the long chemical name: 4-Pregnene-3,20-Dione. That’s progesterone to most folks outside the lab. This compound shows up in hormone research and medicine development, so it finds its way into quite a few labs, not just those stocked with advanced safety gear. The way people handle this powder or crystal often speaks volumes about lab culture, not just chemistry skills.
The first thing I think about isn't glassware or digital balances. I think about hands and lungs. Progesterone isn’t wildly toxic, but it can cause skin irritation and stir up allergic reactions, especially if you handle it often. You fire up the ventilation, glove up, and use the right containers not just because a rulebook said so, but because dust on your skin and in your nose causes real problems down the line. I’ve seen enough red, itchy arms and heard enough sneezes to know that.
Eye irritation concerns me, too. One careless transfer, one gust of air, and chalky dust finds its way onto your eyelids. Most who work with this kind of material keep a bottle of saline handy, not out of paranoia, but out of respect for how fast an ordinary day can turn sideways.
Some lessons stick because they’re about respect—not just for the material, but for the people around you. That’s why I always point out that even trace amounts on a benchtop could transfer onto pens, glassware, or coffee cups. Chemical safety means treating everything as a possible route for exposure, especially when dealing with hormones.
Wearing gloves does more than just protect skin. It stops chemical travel. Nitrile works well, since it stands up to small spills and resists tearing. Putting on lab coats and tying back hair prevents unexpected splashes and accidental contamination.
It’s not enough to just stay clean while working. Hands need a thorough wash with soap and water each time I’m done. I always check that nothing stuck to my watch or the edges of sleeves—which sounds fussy, but stopping hormones from getting on your skin is easier than dealing with unpredictable side effects later.
If I hear the word “ventilation,” my mind goes right to the fume hood. These boxes feel like overkill for something as common as progesterone, but the hood carries away particles that float up and patient, invisible fumes. No fancy tech needed—just working fan and sliding sash. Even on slow days, I never skip the hood, because hormone dust in the air travels through a lab fast.
Storage makes a difference too. I’ve watched people store hormone powders in shared fridges, and all it takes is a leaky cap to cross-contaminate solvents or samples. Glass jars with screw-on lids, labeled in big print, cut confusion and keep everyone on the same page. Storing these jars in a cool, dry place, away from food or drink, keeps accidents to a minimum.
I once learned more from five minutes chatting with an experienced postdoc than from any printed MSDS sheet. People share “good cheat codes” for pouring and weighing—like using parchment paper to keep tablets from bouncing off the scale. When labs make a habit out of talking about hazards, even the most routine day feels safer. Safety grows from direct experience, not just lists of rules. Listening to the people who've handled this hormone a hundred times proves just as important as following the checklist taped above the bench.
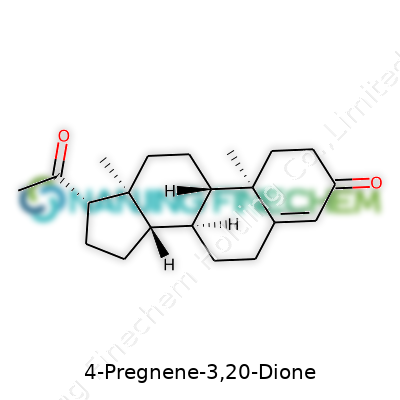
| Names | |
| Preferred IUPAC name | (8S,9S,10R,13S,14S,17S)-10,13-dimethyl-17-(1-oxopropan-2-yl)-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthrene-3,20-dione |
| Other names |
Pregn-4-ene-3,20-dione Delta(4)-progesterone 4-Pregnen-3,20-dione Pregna-4,9(11)-diene-3,20-dione Progesterone |
| Pronunciation | /ˈfɔːr preɡˈniːn θriː twɛn.ti daɪˈoʊn/ |
| Identifiers | |
| CAS Number | 53-03-2 |
| Beilstein Reference | 1302081 |
| ChEBI | CHEBI:28722 |
| ChEMBL | CHEMBL1134 |
| ChemSpider | 6826 |
| DrugBank | DB00629 |
| ECHA InfoCard | ECHA InfoCard: 100.000.085 |
| EC Number | 1.3.99.4 |
| Gmelin Reference | 10406 |
| KEGG | C00514 |
| MeSH | D011317 |
| PubChem CID | 6241 |
| RTECS number | UY7560000 |
| UNII | 7J22P6C9A2 |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID4022077 |
| Properties | |
| Chemical formula | C21H30O2 |
| Molar mass | 314.469 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.12 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 1.87 |
| Vapor pressure | 1.88E-7 mmHg |
| Acidity (pKa) | 12.78 |
| Basicity (pKb) | -3.2 |
| Magnetic susceptibility (χ) | -95.0·10^-6 cm³/mol |
| Refractive index (nD) | 1.0590 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 312.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -372.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6672.5 kJ/mol |
| Pharmacology | |
| ATC code | G03BA03 |
| Hazards | |
| Main hazards | H319: Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P261, P264, P301+P312, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 4°C |
| LD50 (median dose) | LD50 (median dose): Mouse oral 1113 mg/kg |
| NIOSH | PY8050000 |
| PEL (Permissible) | PEL (Permissible) : 15 mg/m3 (total), 5 mg/m3 (respirable fraction) |
| REL (Recommended) | 0.05 mg/m³ |
| Related compounds | |
| Related compounds |
Pregnenolone Progesterone 17α-Hydroxyprogesterone Corticosterone Cortisol Aldosterone |