Chemistry has always thrived on finding the right intermediates for more efficient synthesis. In the late 20th century, chemical companies zeroed in on chlorinated and nitrated benzene derivatives for their potential use in pharmaceuticals, agrochemicals, and advanced materials. 4-Chloro-3-nitrobenzonitrile came into focus around this period as researchers began looking for more reactive and stable intermediates, improving yields in several synthetic processes. The compound’s robust structure and reactivity opened doors for novel reactions that didn’t flourish with more basic compounds, providing a foundation for further innovation in organic synthesis. Professional chemists saw real progress by shifting away from simpler benzonitriles to this more functionalized version, directly impacting the efficiency of reaction steps in multi-step chemical syntheses.
4-Chloro-3-nitrobenzonitrile is a pale yellow or off-white crystalline solid, recognized by its sharp, pungent chemical odor. In labs, this compound is prized as a versatile intermediate, often kept in tightly sealed containers because it reacts easily under specific conditions. The purity often lies above 98%, a necessity for research and industry. Chemists value its unique combination of nitrile, chloro, and nitro groups on a single aromatic ring, which creates several possibilities for further chemical transformations. This versatility ensures it features in both niche and broad-based chemical syntheses, setting it apart from less functionalized benzonitriles.
At room temperature, 4-chloro-3-nitrobenzonitrile presents as a solid, with a melting point generally falling between 130°C and 135°C. It dissolves poorly in water but mixes well with many organic solvents, including DMSO, DMF, and acetone. The compound shows a notable degree of stability, enduring typical lab conditions and moderate heat without significant decomposition. Its nitrile group gives it some polarity, which helps drive specific reactions, while the nitro and chloro substituents increase its electrophilic reactivity, allowing further derivatization through nucleophilic aromatic substitution and reduction reactions. Its boiling point exceeds the range of common organic solvents, so chemists rarely encounter it as a vapor under normal lab use.
Industry often requests detailed technical sheets with every shipment—purity, melting point, identity confirmation by HPLC or NMR, and details on trace residues. The packaging sports hazard labels due to the irritant nature of the compound and its volatility, with warning symbols for both skin contact and environmental harm. Companies add batch numbers and full regulatory compliance details to every container, making traceability easy in case of any quality or safety issue. It’s common to see requests for custom grades, with certain users preferring higher purity for electronics or pharmaceutical applications, where unwanted side reactions cause major headaches.
Modern production usually begins with chlorination of 3-nitrobenzonitrile, using established halogenation techniques under controlled temperature. This process avoids overchlorination by maintaining precise stoichiometry and temperature control. Sometimes, synthesis starts from 4-chlorobenzonitrile with nitration in the presence of strong acids. Both methods demand careful purification steps—recrystallization and solvent washings ensure minimal residual acids, solvents, or unreacted starting material. On an industrial scale, reactors equipped with corrosion-resistant linings handle aggressive reaction media. Disposal of acidic waste calls for strict environmental controls, as required by regulatory law.
The compound reacts with a variety of nucleophiles, growing into other valuable derivatives by swapping the chloro group or reducing the nitro group under hydrogenation conditions. Palladium-catalyzed cross-coupling steps, such as Suzuki or Heck reactions, expand the compound’s utility across fine chemical and drug development projects. Reductions with iron-acid or catalytic hydrogenation furnish the corresponding amine, a precursor for dyes, pharmaceuticals, and specialty chemicals. Functional group interconversions such as hydrolysis or further nitration often underpin synthetic routes tailored for target molecules in R&D and manufacturing. Unlike unsubstituted benzonitriles, these reactions typically proceed under milder conditions, saving both time and energy in the lab.
Suppliers and chemical databases recognize 4-chloro-3-nitrobenzonitrile under several labels: 3-nitro-4-chlorobenzonitrile, 4-chloro-3-nitrobenzenecarbonitrile, and its registry number, CAS 61969-50-4. Always check chemical catalogs since brand-specific names turn up. Local distributors frequently lean on regional naming conventions, but any confusion fades with consistent use of the CAS number during orders or referencing.
Lab users can’t afford to skip gloves or eye protection because this compound irritates mucous membranes and the respiratory tract. Inhalation is risky, so chemical fume hoods remain mandatory. Spills need immediate containment using inert absorbents, followed by proper hazardous waste disposal procedures. Material safety data sheets require review before first use; workers should never shortcut these routines. Storage away from strong bases and reducing agents prevents dangerous decomposition or inadvertent side reactions. Large-scale handlers turn to custom exhaust and scrubbing systems to keep airborne concentrations below workplace exposure limits.
Fine chemicals, drug intermediates, dyes, and advanced polymers draw on this compound thanks to its reactive positions and functional group array. Pharmaceutical engineers use it to build heterocyclic structures and active drug ingredients. Agrochemical developers incorporate its scaffold into selective herbicides. Materials researchers covet its potential for electronic and high-performance coatings where precise functionality in the aromatic ring counts. Academic groups design new coupling and functionalization reactions with this molecule at the center to push boundaries in organic synthesis or catalysis.
Chemists strive to exploit every facet of 4-chloro-3-nitrobenzonitrile’s structure. Modern R&D projects test greener ways to create and modify this compound, such as moving away from harsh acids or expensive metals for basic transformations. Development teams also look for more selective catalysts and alternative solvents that reduce hazardous waste. Analytical chemists develop new spectral methods for better impurity profiling, vital for high-purity requirements. The compound frequently pops up in patent filings for both pharmaceutical and materials innovations, reflecting its continuing role as a building block.
Toxicology teams run a battery of tests, taking a close look at how this compound affects lab animals and cell cultures. Direct skin contact leads to irritation, and chronic exposure raises more concern. No scientist likes to take risks with aromatic nitro compounds, since past studies link related structures to mutagenicity and metabolic byproducts. Waste handling guidelines ask for neutralization followed by licensed disposal to protect both workers and downstream environmental systems. Ongoing studies explore breakdown products, bioaccumulation risk, and effective remediation strategies inside and outside the lab.
Researchers and chemical engineers see new opportunities in expanding 4-chloro-3-nitrobenzonitrile production from renewable feedstocks, slashing the carbon footprint in line with global climate goals. Industrial chemists work on continuous flow setups with precise automation for higher yields and fewer byproducts. Material scientists design next-generation performance coatings and advanced organic semiconductors using this compound as a lead intermediate. The full impact unfolds as R&D uncovers more efficient uses and eco-friendlier transformations, broadening the compound’s reach into sectors that depend on bespoke aromatic chemistry.
Shaking a jar of 4-Chloro-3-Nitrobenzonitrile in a lab, you’d expect a lot from what looks like a basic yellowish powder. Its purity shapes the outcome of reactions, influences safety, and determines how many headaches you’ll face later on during synthesis and analysis. In most catalogs or chemical databases, you’ll spot this compound with a listed purity around 97% to 99%. The highest grades seen in commercial lots rarely go beyond this range because producing ultra-pure batches hikes up the cost and effort for something only a slice of the market truly requires.
Using low-grade chemicals early on, especially in a research project, often leads to second-guessing results. Anyone who’s tried running a reaction with unknown contaminants can recall the head-scratching moments at the fume hood, wondering if that faint TLC spot came from your desired product or something that snuck in due to impurities. Even a couple percent of byproducts in this chemical, such as other nitrobenzonitrile isomers or unreacted starting materials, spark extra troubleshooting steps, slow purification, or worse, misleading conclusions. Imagine troubleshooting for weeks, only to realize that the issue sprouted from a cheap purchase on a 95% batch.
As an intermediate in pharmaceuticals or advanced materials, impurities in 4-Chloro-3-Nitrobenzonitrile can linger in finished products. Nobody wants a trace of leftover trichloro or dinitro byproducts in a drug candidate, not after months of work. Quality control faces an uphill battle battling background signals or shadow peaks in HPLC runs, chasing low-level contaminants that keep slipping through. So, even if your supplier’s certificate claims “min. 98%,” real-world batches sometimes drop below that after months in a warehouse or after a few cycles of taking scoops for different experiments.
I’ve ordered this compound from multiple vendors and seen variation batch to batch. Factors influencing purity boil down to how each vendor crystallizes and dries the product, and how strictly they screen for residual organics or heavy metals. If a company cuts corners on these steps, their product smuggles in extra junk. This happens more in bulk supply destined for industrial settings, where low purity is more tolerable, versus high-stakes pharmaceutical labs where every step demands a higher grade.
If you want reliable results, spring for a certificate of analysis and ask for recent chromatography. Check for listed percentages, not blanket “technical grade” or “pure.” Strict regulatory fields—think drug development or substrate manufacturing—won’t accept anything below 98% without extensive extra purification. For exploratory or non-critical experiments, a 95% batch may do, but will likely require extra filtering or crystallization.
Researchers stuck with questionable lots can turn to common tricks: recrystallization, washing, and sometimes even vacuum sublimation. These aren’t always foolproof but give better odds than rolling the dice with a cheap stock. Labs with tight budgets can sometimes collaborate for a group buy of higher-purity materials, driving the price down while boosting consistency. Documenting lot numbers and reporting back to suppliers can pressure vendors to uphold higher standards. In the end, the effort put into chasing a clean batch early can save months of chasing ghosts in follow-up work.
My first experience unpacking a shipment of specialty chemicals for an R&D lab hammered home the point: not every powder gets tossed on the same shelf. Even small mistakes can lead to wasted product, ruined experiments, or worse, safety risks. 4-Chloro-3-Nitrobenzonitrile falls into the group of compounds that don’t forgive negligence.
This compound carries two warning flags—nitro and nitrile. The nitro group often signals reactivity, especially near heat or light. The nitrile demands a shield from moisture and strong bases. These features make the molecule useful but require respect—just like an old reactor’s pressure gauge you check twice.
Moisture ranks high among enemies. Too much humidity spells trouble, often nudging hydrolysis or slow decomposition. I’ve seen stubborn clumping and even color change when left near a steam line. A dry cabinet or desiccator earns its keep in the storeroom.
Steering clear of sunlight makes just as much sense. UV can excite nitro compounds, driving slow photochemical changes that ruin purity. A brown glass container or anything opaque blocks most of the harmful rays. It seems simple, but one forgotten vial by a window can turn an expensive sample into waste.
Heat always stirs up problems, and this chemical plays by the same rules. Temperatures hovering around room level suit most benzonitrile derivatives, but I prefer sticking closer to 15–25°C. Anything above starts to boost vapor pressure and push along side reactions. Chemical catalogs don’t list a need for a fridge, just a stable shelf away from radiators or ovens.
Glass beats plastic for long-term storage. Cyano and nitro groups sometimes react with plasticizers, and a cracked container creates a risky mess. Screw caps with PTFE liners seal out air and water vapor. I once saw a cheap plastic lid swell, leaking solvent fumes—the lesson stuck. Anyone who’s fished melted ring binders out of the chemicals bin knows a good container pays for itself.
Label everything. Sharpies fade; chemical-resistant labels never do. A half-life of unlabeled powders in a busy lab drops fast. Segregation also keeps everyone safer—never stick aromatic nitriles next to acids or strong bases. Acid fumes corrode caps and sometimes attack the compound itself.
Spill response plans get ignored until things happen. Simple absorbents, a splash of sodium bicarbonate, and a clear run to the sink can prevent a small accident from going sideways. Even outside of accidents, frequent cleanouts make it easier to spot odd smells or clumpy stocks before they become big problems.
Use tight, labeled glass bottles with a secure seal and park them in cool, dry cabinets, ideally with a handful of desiccant bags. Avoid light, steam, and temperature swings. Make organization a habit—sorted by hazard class rather than alphabetically. Train new staff to see storage as a critical skill, not busywork. Memorize where to find the material safety data sheet before you need it. If budgets allow, invest in a locked chemical safe and regular audits; no one likes surprises during inspections.
Setting up these simple routines makes life easier not just for safety managers or inspectors, but for anyone who relies on these chemicals for solid, reproducible work. Every bottle kept tight and cool becomes one less headache down the road.
4-Chloro-3-Nitrobenzonitrile might sound like something only chemists bring up, but its footprint stretches across a surprising range of industrial corners. Walk through a pesticide plant or flip through patent filings on pharmaceuticals, and this compound keeps popping up. A glance at its structure—a benzene ring sporting a chlorine, a nitro, and a nitrile group—tells a story of reactivity and utility. It’s not sitting on a shelf gathering dust; it’s a workhorse in some of today’s most important chemical processes.
Farm fields matter as much to me as the grocery aisle prices that shape everyday budgets. Growing up in a rural community, you realize that keeping crops healthy isn’t just about good weather; it comes down to the chemistry behind pesticides and herbicides. 4-Chloro-3-Nitrobenzonitrile is regularly tapped as a key building block for making both. Its reaction profile fits what agrochemical firms look for in manufacturing intermediates, fueling synthesis routes that create effective crop protection chemicals. These aren’t boutique products—they fill warehouses and touch millions of acres every year. With global food demand always on the rise, new molecules built off this compound continue drawing investment and lab attention.
Hospitals, pharmacies, and even basic first-aid kits benefit from the hard work that happens in industrial reactors. I remember visiting a pharmaceutical plant during a college internship and seeing just how intricate each production step could be. 4-Chloro-3-Nitrobenzonitrile carves out a place here, showing up as a starting point for synthesizing active ingredients in both experimental and established medicines. The combination of electron-withdrawing groups on the ring sets this compound up as a smart choice for making other functional molecules. Think of anti-inflammatory drugs, some cancer therapies, and specialty treatments that require finely tuned chemical backbones.
Vivid fabrics and colorful paints draw on chemistry that often gets overlooked. My own run-in with the dye industry, helping a friend set up a small textile business, showed me the importance of reliable intermediates. 4-Chloro-3-Nitrobenzonitrile lends itself to making certain azo dyes and pigments. Its structure supports the addition of various functional groups through controlled reactions, impacting the shade, stability, and even lightfastness of products lining store shelves. These aren’t only for fashion; they show up in industrial coatings, plastics, and even ink for printers.
Every industrial mainstay faces growing pains and changing rules. The production and handling of 4-Chloro-3-Nitrobenzonitrile involves safety and environmental questions. Breathing in dust or improper storage can put workers at risk—a fact that’s led to tighter workplace standards. As more people pay attention to green chemistry, the industry is looking for ways to cut waste from synthesis routes and find less hazardous solvents. Cleaner reaction conditions and better recycling will help ease pressure on both people and the planet. Cutting-edge research teams at universities and major manufacturers are pushing hard here, and there’s slow but steady progress worth watching.
Despite sounding foreign to most ears, chemicals like 4-Chloro-3-Nitrobenzonitrile shape daily life in unexpected ways. They sit behind affordable food, accessible medicine, and everyday color. Seeing industrial chemistry up close doesn’t solve every supply chain issue or safety concern, but it gives a sense of just how much depends on a molecule few ever see.
4-Chloro-3-Nitrobenzonitrile sounds like something you’d find in a graduate chemist’s thesis, but for industries ranging from pharmaceuticals to specialty polymers, this compound plays a surprisingly crucial role. Over the past decade, there’s been a rising interest in its use, thanks to its function in chemical synthesis. The big question getting tossed around now—can you actually buy it in bulk if you’re not some gigantic multinational?
Anyone who’s ever tried to source specialty chemicals knows the headaches that come with scaling. I remember listening to a friend moan about waiting three weeks on a shipment from China, only to find his supplier ran out halfway through the order. Most big suppliers—think Merck, TCI, Sigma-Aldrich—treat 4-Chloro-3-Nitrobenzonitrile as a catalog item, so you’ll often see it listed in neat little grams or maybe a bottle or two at 100g each. That’s not how factory floors or research facilities work. People in these industries need kilos, sometimes even drums, not hobbyist bottles.
Demand isn’t just a matter of academic curiosity. This compound feeds directly into making several types of active pharmaceutical ingredients and advanced agrochemicals. In one example, fine chemicals like this serve as intermediates for making antihypertensive drugs or crop-protection agents. The more our population grows, the more pills and pesticides get manufactured. Companies know this, which is why sourcing managers watch global prices for compounds like 4-Chloro-3-Nitrobenzonitrile nearly as closely as corn or copper—every disruption can lead to missed production deadlines.
Bulk availability almost always comes down to two simple things: supply capacity and regulatory paperwork. China and India dominate the market for intermediates. Many smaller chemical companies run production lines that can churn out hundreds of kilos, so technically, yes, you can get bulk quantities. But here's where reality bites. Import laws, especially after COVID-19 supply chain chaos, mean smaller buyers end up competing with larger firms for the same kg stockpiles. Customs sometimes hold shipments for weeks on end, while some Western countries have stricter purity requirements, and not every bulk batch meets them.
If you’re hunting for bulk, prepare to deal directly with manufacturers instead of distributors. The big distributors will probably quote you a steep markup or say they’re “out of stock,” pushing you to go straight to the source—usually in Asia. I’ve watched companies spend weeks just negotiating specs and logistics for an order measured in kilograms. This industry doesn't encourage spontaneity.
None of these roadblocks are insurmountable, but they force buyers to rethink their approach. If you need bulk quantities, you’ll end up building personal relationships with suppliers. Visiting plants, checking their quality controls, and running your own lab tests help cut down on the risk that an order will be delayed or subpar. Firms also try to diversify—working with more than one supplier prevents a single missed shipment from stalling operations. Sometimes, groups of midsize buyers form consortia to reach bulk price points together and wield more bargaining power.
These are the realities for anyone looking to bring in 4-Chloro-3-Nitrobenzonitrile in more than a token amount. Getting the right quantity, on time, at spec, is less about clicking “order” and more about shaking hands—virtually or in person—with the right people.
In chemistry labs, every bottle carries a number. That number, the CAS number, never lies about what’s inside. For 4-Chloro-3-Nitrobenzonitrile, the CAS number is 21409-49-8. Written on labels, invoices, and shipped containers, this sequence becomes a quick handshake of trust between scientists, buyers, and regulators around the globe. Numbers like this cut through the fog of similar-sounding chemicals, avoiding mix-ups that can spell trouble for research, safety, or business.
I’ve watched people try to manage projects with only chemical names guiding them. Let’s just say the potential for error jumps off the charts. Common names sometimes twist in translation, formulas get jumbled, and before long, someone orders the wrong compound. The CAS number locks in the identity of 4-Chloro-3-Nitrobenzonitrile no matter how anyone phrases it, whether they’re in New Jersey or New Delhi. That kind of clarity carries a huge weight in pharmaceutical development and industrial supply chains.
Big manufacturers rely on these numbers in their ordering systems. Smaller labs, always pressed for time and budgets, skirt disaster by checking CAS numbers before mixing or reacting anything. Older colleagues used to mention how some chemicals picked up entirely new nicknames over time, muddying things for the next generation. Relying on the CAS number builds a bridge across countries, companies, and decades.
Walking into a warehouse with a clipboard, people get bombarded with bags and drums full of hard-to-pronounce names. The only surefire anchor becomes that CAS number. Not every supplier is reputable and the counterfeit chemical market has crept into many countries. A label mix-up here does not stand as a mere paperwork nuisance—it carries risks to product quality, safety, profit, and even public health.
The world saw the consequences of identity mix-ups during the pandemic, when many manufacturers scrambled to track raw chemical origins. The CAS registry stepped up to slice confusion. Factories that stuck to strict CAS matching had far fewer hold-ups and quality control checks. There is something strikingly human about using a simple number to cut through chaos and uncertainty so regular people don’t have to worry about the safety of their medicine or materials.
Clearing up confusion about things like 4-Chloro-3-Nitrobenzonitrile often means nudging teams to treat the CAS number as their main reference. Some colleagues push back, worrying about the loss of familiar names. Yet making the switch leads to less scrap, smoother audits, and better collaboration. Building good habits in the lab—from double-checking that “21409-49-8” matches on an incoming barrel, to listing the CAS with every internal order—makes the work smarter, safer, and less stressful.
If there’s one lesson that sticks, it’s that precision matters every step of the way. With so many chemicals only a letter or digit apart in name or formula, anchoring decisions to the CAS number has no downside. Real quality depends on details like these, easily overlooked but vital in the world most of us rarely see up close.
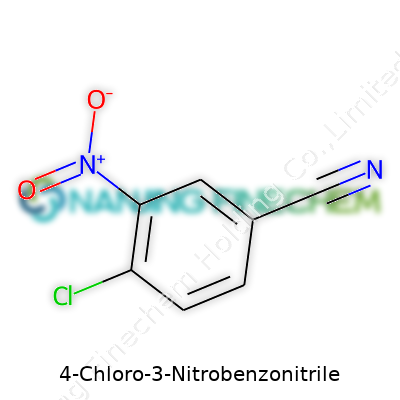
| Names | |
| Preferred IUPAC name | 4-chloro-3-nitrobenzenecarbonitrile |
| Other names |
3-Nitro-4-chlorobenzonitrile 4-Chloro-3-nitrobenzenecarbonitrile 1-Chloro-2-nitro-4-cyanobenzene |
| Pronunciation | /ˈfɔːr ˈklɔːr.oʊ ˈθriː ˈnaɪ.troʊ ˌbɛn.zoʊˈnaɪ.trəl/ |
| Identifiers | |
| CAS Number | 13799-35-8 |
| Beilstein Reference | 1721487 |
| ChEBI | CHEBI:91541 |
| ChEMBL | CHEMBL161541 |
| ChemSpider | 211710 |
| DrugBank | DB07712 |
| ECHA InfoCard | ECHA InfoCard: 100.018.142 |
| EC Number | 610-473-5 |
| Gmelin Reference | 1621036 |
| KEGG | C19238 |
| MeSH | D09. Chemicals and Drugs Category |
| PubChem CID | 102395 |
| RTECS number | GE8400000 |
| UNII | 15G36M46J1 |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C7H3ClN2O2 |
| Molar mass | 197.56 g/mol |
| Appearance | Light yellow to yellow crystalline powder |
| Odor | Odorless |
| Density | 1.53 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.98 |
| Vapor pressure | 0.00011 hPa (25 °C) |
| Acidity (pKa) | 1.39 |
| Basicity (pKb) | 1.44 |
| Magnetic susceptibility (χ) | -63.0e-6 cm³/mol |
| Refractive index (nD) | 1.629 |
| Dipole moment | 3.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 220.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −33.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -573 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 3-0-2-Health |
| Flash point | 113°C |
| Lethal dose or concentration | LD50 Oral Rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | CN9175000 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | REL: 0.3 mg/m³ |
| Related compounds | |
| Related compounds |
3-Chloro-4-nitrobenzonitrile 4-Chloro-3-nitrobenzaldehyde 4-Chloro-3-nitroaniline 4-Chloro-3-nitrobenzoic acid 4-Chloro-3-nitrotoluene |