Chemistry likes to throw together molecules in search of properties that push science forward, and 3-Amino-4-Nitrobenzonitrile grew out of that restless curiosity. Decades ago, researchers poked around with nitrated aromatic compounds to satisfy pharmaceutical, dye, and intermediate markets. Labs across Europe and the US found themselves drawn to these ring-based molecules for their promise in synthesizing more complex materials. The push to develop better antibiotics and colorants during the mid-20th century pulled attention toward nitrile derivatives, with 3-Amino-4-Nitrobenzonitrile showing up as a byproduct and slowly carving out a spot for itself in technical catalogs. Its story is littered with patents, as clever chemists worked out new routes, modifications, and shortcuts—a reminder that trial, error, and a willingness to work with finicky substances keep chemistry moving forward.
Labeled as both a specialty intermediate and research chemical, this molecule attracts demand from fine chemical manufacturers, pharmaceutical labs, and pigment producers. Comes as a yellow powder, sometimes a light orange if the synthesis runs hot. Its trade value hinges on purity, consistent color, and ease in further reactions—chemists get impatient with mixtures and impurities, so suppliers who lock down their process earn repeat calls. Online catalogs often lump this compound into the “rare and in-stock only if you’re lucky” section, driving its cost higher compared to simpler benzonitriles. It’s not a commodity chemical; smaller research and manufacturing outfits tend to order it in grams or kilos rather than drums.
Appearance-wise, you get a crystalline solid, yellowish-brown—not something you’d mistake for a food additive. It displays a decent melting point, generally around 140-144°C, settling just where aromatic nitro groups tend to place it. Solubility leans toward organic solvents; acetonitrile, DMSO, dimethylformamide, and hot ethanol work well to dissolve it, while water barely gives it a glance. The molecule (C7H5N3O2) weights in at roughly 163 grams per mole, and packs a nitro group and an amino group onto the benzene ring, making it a versatile foundation for further reactions. In air, the powder remains stable in closed containers, but direct sunlight or moisture starts to wear away at its quality.
Product labels always highlight CAS number 619-46-7, purity percentages, batch number, and storage notes. Any supplier worth ordering from ships it with HPLC/GC data showing at least 97% assay, and impurity levels down to tenths of a percent. Safety sheets attach themselves to every shipment, spelling out risks so nobody’s caught unprepared. The label is often bilingual or trilingual to serve the international market. For lab compliance, hazard pictograms—explosive-looking exclamation marks and environmental hazard icons—make the risks impossible to miss.
The prep walk-through isn’t cozy, because you need precision. Most syntheses start from 4-nitrobenzonitrile, which reacts with ammonia gas under pressure (or, for those less equipped, concentrated ammonium hydroxide in a sealed vessel with a catalyst). Reaction conditions dance around 80-110°C. Some chemists take the indirect route—nitrating 3-aminobenzonitrile with a controlled dose of nitric acid and keeping temperatures low to prevent over-nitration and degradation. Each approach brings trade-offs between yield, safety, and raw material costs. Side reactions, particularly dinitro products, require snappy purification steps, usually recrystallization or filtration through activated carbon. Synthesis at scale calls for strong ventilation, pressure-rated equipment, and patience with the messiness of organic powders.
3-Amino-4-Nitrobenzonitrile opens up plenty of reaction options. The amino group serves up nucleophilic spots for acylation, sulfonation, or condensation, leading to ureas, amides, or more complicated ring structures. The nitro group can get reduced to an amine with the right reducing agent, such as tin(II) chloride, iron filings, or catalytic hydrogenation, creating diamino derivatives valuable for dye chemistry or advanced synthesis. The nitrile can hydrolyze into a carboxylic acid under tough alkaline or acid conditions, but most reactions treat it as a stable stub, letting chemists tinker with other functional groups without worrying about side breakdown. Plenty of patents recommend its use for coupling, cyclization, or as a step in the buildout of pharmaceutical scaffolds.
The world of chemistry packs multiple names for the same compound: 3-amino-4-nitrobenzonitrile, 4-nitro-3-aminobenzonitrile, m-amino-p-nitrobenzonitrile, and even abbreviated as ANBN by those tight on report page space. Some catalogs push it under trade names like NABC or AN BENZONITRILE, keeping it visible only to specialized buyers who already know what they’re hunting for.
Working with this compound calls for sturdy lab routines. It won’t outright explode at room temperature, but that nitro group raises eyebrows in safety audits. Gloves, goggles, and masks drop the risk of dust inhalation or skin contact, and fume hoods pull away aggressive vapors if solvents get involved. Disposal rules kick in because nitroaromatics create headaches at waste treatment plants—burn it, and the fumes can turn toxic; flush it, and the groundwater gets unfriendly. European REACH regulations and US OSHA rules round up the necessary safety data and labeling, so shops and universities keep their compliance teams in the loop. Most real-life accidents come from rushed work or skipping PPE, not freak properties in the molecule itself.
This compound winds up tucked away in the middle stages of pharmaceutical manufacturing, pigment and dye synthesis, agrochemical ingredient prep, or specialty material design. Drug researchers covet it for building heterocyclic compounds—antibacterials, antidiabetics, and even anti-tumor candidates have relied on this scaffold. Dye chemists exploit its dual reactivity to make azo or anthraquinone structures, giving fabrics and paper durable color. A handful of electronics manufacturers dip into nitrile intermediates for crafting organic switches and sensors, though these uses never rely on mass quantities.
Academic labs tinker with new reactions using 3-Amino-4-Nitrobenzonitrile, looking for cleaner, faster, or greener processes. As green chemistry grabs more headlines, researchers try swapping out old nitro group additions with safer alternatives that lower byproduct waste and energy use. Pharmaceutical companies set up structure-activity relationship studies (SAR) using benzonitrile cores, testing hundreds of derivatives for bioactivity, absorption, or metabolic stability. Several articles in international journals give this molecule a starring role in new synthetic methods, such as microwave-assisted or solvent-free protocols that aim to speed up reaction times without sacrificing product yield. At the same time, analytical chemists work up new detection routines for tracking trace levels in finished products or environmental samples, keeping safety regulators up to speed.
Most benzonitrile derivatives get handled with suspicion, since aromatic nitro groups have a reputation for toxicity. Rat and mouse studies detail moderate acute toxicity through ingestion and chronic impacts through repeated exposure. Lab workers occasionally report mild skin irritation, headaches, or dizziness from fume exposure, especially in small, poorly ventilated settings. The nitro group heightens the risk of methemoglobinemia in mammals, potentially linking long-term exposure to blood or nerve disorders. Environmental breakdown proceeds slowly, with the compound sticking around in soils and waterways, raising concerns about bioaccumulation if disposal isn’t managed well. Regulatory agencies lean on published animal data for guidance, and workplace exposure limits usually fall well below one milligram per cubic meter.
The story of 3-Amino-4-Nitrobenzonitrile keeps going as chemists crank out new drugs and materials that need it in their ancestry. As the world swings toward stricter safety and environmental rules, future production will depend on cleaner manufacturing routes, tighter quality standards, and improved disposal and recovery processes. Green chemistry’s influence nudges research toward safer nitration and amination steps, pushing suppliers and buyers alike to shift away from traditional, hazardous reagents. Discoveries in medicinal chemistry may open up new demand for its derivatives, keeping it relevant in small but vital corners of industry and science. If new applications in electronics or sustainable dyes materialize, demand could nudge up, but the heart of its utility stays fixed to research, flexibility, and the willingness of chemists to adapt as tools and expectations shift.
3-Amino-4-nitrobenzonitrile carries the formula C7H5N3O2. Its molecular weight lands right at 163.14 g/mol. These numbers speak louder than most folks realize. Those twelve atoms—seven carbons, five hydrogens, three nitrogens, two oxygens—might look modest, but they build a compound that turns up in more places than most people encounter in a lifetime.
My own days in the lab taught me a lot about why knowing a formula and weight matters. In college, not long after I first held a pipette, I found myself troubleshooting a synthesis that kept falling short of yield predictions. What solved the problem didn’t come from some fancy technique, but from sitting down and really looking at the formulas and weights on the page—basic but powerful. Precision turned things around, and I walked away respecting those basic numbers.
Getting the chemical identity right shapes everything in a research project. That molecular weight isn’t just trivia—it tells you how much to weigh out, how to plan a reaction, and how to keep things safe. The formula pulls back the curtain on what’s possible; one misplaced atom can turn a useful building block into a red herring.
I remember a grad student working next to me who once switched up nitro and amino groups by mistake—paperwork and folderol followed because the wrong intermediate entered the workflow. It wasted time, money, and a lot of good will. Little atoms, big consequences.
3-Amino-4-nitrobenzonitrile can stump even skilled hands. The nitro group plays tough during reduction, and the lone nitrile can slip through purification steps. Skipping the math only leads to frustration. Labs working with new drug leads or dye intermediates often need sharp attention to each atom. Without it, reactions stall, progress halts, and risk creeps in.
The answer lies in going back to basics. Every chemist I know learns early that shortcuts unravel fast. Tallying up the formula and weighing each batch with intent can save months of setbacks. Modern tools help—a good analytical balance, a solid NMR or MS readout, double-checking entries before following a synthetic procedure. Peer review inside the lab, even if it’s just a quick look from a colleague, weeds out mistakes before they grow roots.
In schools, better training could make these concepts sink in deeper. When students touch real chemicals instead of just seeing names on a board, they understand that a single digit in a formula changes everything.
The story of 3-amino-4-nitrobenzonitrile goes beyond theory. Chemicals like this enter paints, medicines, and electronic parts. Workers from more than just academic labs deal with molecules like it every day. The groundwork—the formula, the weight, the structure—keeps everyone working safely and effectively. Small details matter, and even a pocket calculator can make all the difference.
I’ve crossed paths with all sorts of specialty chemicals over the years, so spotting 3-Amino-4-Nitrobenzonitrile on a product list never raised eyebrows at first. Yet when you lift the hood, this compound delivers some surprising utility far beyond a basic catalog number. At a glance, you notice it’s not some household staple, but it quietly supports a range of industries people rely on every day.
Drug discovery teams lean on 3-Amino-4-Nitrobenzonitrile for the sheer flexibility it gives in building complex molecules. Its structure acts as a backbone, with the amino, nitro, and nitrile groups each ready to jump into action. These pieces make it handy for synthesizing more advanced pharmaceutical ingredients—painkillers, antivirals, and even some anti-cancer compounds often trace a portion of their development back to chemical blocks like this. While the nitrile function locks in place, the amino and nitro groups bring reactivity, letting researchers tweak them as needed and slot the results into larger drug frameworks.
Anyone who's ever worked in plastics, paints, or textiles knows color matters. Here, 3-Amino-4-Nitrobenzonitrile serves as a building stone for making vibrant, long-lasting dyes and pigments. Its chemical frame holds colors tightly and resists fading, something you notice most in high-wear materials like clothes, automotive parts, or outdoor signs. Companies count on its performance where bright, durable colors make a difference—whether that’s in packaging that jumps off the shelf, or uniforms that outlast countless washes.
This molecule isn’t just a one-trick pony. On factory floors, it’s a classic intermediary, standing in as both a starting point and a problem solver. In agricultural chemistry, for instance, it helps shape new herbicides and pesticides. It’s not usually the ingredient ending up in your tomato patch, but it gives chemists the flexibility to attach other functional groups, explore new modes of action, and tune performance. This kind of tinkering allows for safer, more effective crop protection—a goal anyone with a stake in farming can appreciate.
Many specialty chemicals fill only a niche, but this compound keeps showing up across various fields for good reasons. Its versatility opens doors for innovation, whether someone’s chasing a tougher pigment or the next breakthrough in pain management. Sure, not many people outside R&D see the name pop up, but society feels the ripple effects—better medicines, more rugged consumer goods, and less pesticide on fields, all thanks to chemical blocks like this.
Handling and sourcing specialty intermediates like 3-Amino-4-Nitrobenzonitrile can challenge both manufacturers and regulators. Supply chains get tangled, and prices sometimes swing wildly. So building secure supplier relationships, checking regulatory compliance, and doubling down on greener synthesis routes go a long way toward smoothing out the process. Real progress means investing in research that uses cleaner catalysts or renewable feedstocks—steps that shrink the environmental footprint. Companies that keep their eyes on both safety and innovation stay out in front, and so do their customers.
This compound shows how thoughtfully designed molecules unlock bigger changes. It’s easy to overlook names buried in technical documents, but their fingerprints linger everywhere—from the bottles in your medicine cabinet to the colors standing the test of time on your favorite shirt.
A lab isn’t a kitchen—cutting corners with chemicals such as 3-Amino-4-Nitrobenzonitrile lands people in trouble pretty fast. This compound, used in dye research and pharmaceutical formulation, asks for real respect when it comes to storage and handling. I’ve worked in enough research spaces to see how a single mistake can cause a shutdown or, worse, harm. Overlook proper storage, and you put both your samples and your team’s health at real risk.
Safety starts plainly with a closed container. That basic step shields this powder from moisture in the air—there’s no need for fancy climate control if the bottle clamps tightly shut and sits away from heat. Humidity sneaks into loose lids, and over time, powders pull in water vapor, encouraging clumping or even changing how the chemical behaves in reactions. Once I grabbed a poorly capped sample and ended up with a hard block instead of the fine, workable powder we needed.
Heat is another big villain. Placing a jar near a radiator or in sunlight is asking for breakdowns in the compound. Stable room temperature, somewhere cool if possible, forms the sweet spot for 3-Amino-4-Nitrobenzonitrile. I remember a colleague who dried his hands above a chemical bench lamp, then put the bottle back right next to it. That whole batch went bad after a month, wasting weeks of planning.
Chemists say “PPE” a hundred times a day but for good reason. Gloves—nitrile or latex—matter, since skin contact with 3-Amino-4-Nitrobenzonitrile can irritate badly or trigger allergies. Lab coats and goggles round out the basics. Plenty of folks skip goggles for quick tasks, but I’ve seen powders puff out during weighing, sending prompt irritations straight into the eyes.
Safety Data Sheets tell the bare minimum, but realities in a working lab throw up curveballs. Spills don’t clean up with a paper towel—containment means dedicated absorbent pads and disposal boxes, all ready in advance. I always stash a cleanup kit nearby, well-stocked before tasks start, not as an afterthought. Sweeping powder or vacuuming just spreads it around—damp cloths, slow motion, and then closed, labeled waste containers prevent headaches down the line.
There’s more to breathing space than cracking open a window. Any manipulation of powders happens under a fume hood, not on the open bench. These hoods move low levels of fumes or fine particles safely away from your face and hands. Even a tiny cloud can linger and drift, which you only realize after everyone in the room starts coughing. That memory of a mild cough and red eyes tells me fume hoods are worth the wait in line every time.
Every space relies on culture, not just rules. Regular checks on storage containers, lab reminders to restock PPE, and practice drills for spills win more than complicated lab manuals. I’ve seen places with those habits avoid accidents, reduce downtime, and work with fewer distractions. Borrowing these habits, not just procedures, turns dry safety protocols into actual protection for both people and research.
Handling 3-Amino-4-Nitrobenzonitrile isn't just a minor affair. People who spend time in labs know that many chemicals bring an element of danger, and this one doesn't shy away from that. Eye, skin, and respiratory irritation stand out as the most noticeable effects. Anyone inhaling a puff or catching some dust in the eye learns fast that goggles and gloves aren’t optional, they’re required. Skin contact can bring rashes or burns, and people with sensitive skin often feel it worse.
Swallowing or breathing in small amounts can bring sudden headaches or dizziness. For anyone who's ever had to dash for fresh air after a spill, the lesson is clear: ventilation isn’t just comfort; it’s safety. Fume hoods keep harmful particles away from lungs and eyes, and they should run every time this compound gets measured, weighed, or mixed into solutions.
Improper storage creates a recipe for accidents. The nitrile and nitro groups in this material raise the risk of long-term health trouble if someone ignores repeated exposure, and everyone who works with it needs to know that improper sealing or careless stacking on crowded shelves can lead to powerful fumes escaping. Cramming bottles together or skipping routine label checks leads to confusion and, worse, mistakes in emergencies.
Disposal of waste requires thought, too. Tossing this chemical into a regular garbage bin or washing it down the sink shouldn’t cross anyone’s mind. Specialized chemical waste collection not only stays within the law, it prevents contamination of groundwater and public systems. Labs following these protocols see fewer accidents and less need for remediation, which keeps everyone safe.
Solid habits make a big difference. In my own work, I’ve seen how a culture of safety, not just a checklist on the wall, changes attitudes. People look out for each other. Training new staff includes actual demonstrations — not just signing a paper but handling the chemical, cleaning up a simulated spill, and finding the eye-wash station while wearing gloves and goggles. That way, in real situations, panic doesn’t take over when something goes wrong.
Preparation also includes having spill kits within arm’s reach. The few times I’ve seen a bottle knocked over, a ready-to-grab absorbent and clear instructions helped avoid a disaster. Emergency showers shouldn’t collect dust. In practice, labeling never just means a marker on a bottle — that label gets backed up by documentation, with everyone knowing where to find data sheets in a pinch.
Some will try to bypass protective steps, thinking they’ll save time or avoid hassle. In the long run, skipping safety opens the door to injuries, lost work days, or worse. No research project deserves taking chances with personal and team health. Building a habit of using the right gear, prioritizing cleanliness, and enforcing clear protocols doesn’t just tick off boxes — it keeps people healthy and projects on track. Anyone working with chemicals like 3-Amino-4-Nitrobenzonitrile should look past convenience and take pride in safe, responsible practice every day.
3-Amino-4-Nitrobenzonitrile comes up a lot in my work with chemicals for dye and pharmaceutical projects. You can’t just grab any batch off the shelf; purity sets the foundation for how well reactions will go and what kind of by-products get left behind. When you look for this compound, you’ll see a range of purity levels. If you need to synthesize specialty dyes or pharmaceutical intermediates, the conversation pretty much settles around 97% purity or higher. Lower grades can muddy up syntheses or waste precious time with extra purification steps.
Digging through supplier catalogs and lab reports, you often find 98% or 99% as go-to choices for demanding applications. Anything below that risks introducing too much nitro or amino by-product, and I’ve seen what a pain that can become in sensitive processes. Analytical summaries from trusted brands spell out trace elements and moisture content, so checking those details before ordering makes a world of difference. For more routine industrial processing, 95% can suffice, though every process engineer has their threshold based on what’s downstream.
People outside the lab rarely think about packaging, but for me—handling powders with strict storage requirements—it means a lot. The stuff reacts with moisture, sunlight, and heat. So, packaging isn’t some afterthought. You’ll spot 3-Amino-4-Nitrobenzonitrile packed mainly in sealed, light-resistant containers—high-density polyethylene bottles, aluminum foil bags, or drum-style fiber containers for bigger runs. Not only do these keep contaminants out, they make life easier for weighing and transferring in the fume hood.
Ordering in kilogram lots means companies offer pail-size drums lined in polythene. For smaller, research-scale use, 100-gram and 500-gram plastic or glass bottles are common. I once received a shipment in a plain zip bag, and the result was a clumpy mess from humidity. Sealed, tamper-evident caps give you confidence that you’re not working with degraded material. For high-purity work, you often find bottles double-bagged inside a cardboard box with desiccant pouches tucked in.
With chemical prices what they are these days, ordering oversized drums wastes money and shelf space. In our lab, we prefer ordering smaller bottles to cut down on exposure each time the container’s opened. Manufacturers who offer a wider range of options—multiple bottle sizes, bulk drums, vacuum-sealed pouches—win points in my book. Smaller, well-sealed containers make inventory simpler and reduce the odds of cross-contamination. Cost-conscious labs can pool resources with nearby teams or coordinate orders to avoid paying for shipping half-empty containers back and forth.
I’ve seen labs overlook these basics and pay a price. Purity slips, unwanted by-products show up, and that means lost time. Storage headaches follow from flimsy packaging; somebody inevitably has to remake a batch or toss clumped old powder. I always suggest researchers work directly with suppliers to get the certificates that spell out actual content, not just catalog spec sheets, and to sort out the best practical size for their own application. It keeps costs in line and makes the day-to-day job a little less stressful, which in my experience, adds up to smoother projects—and better results in the end.
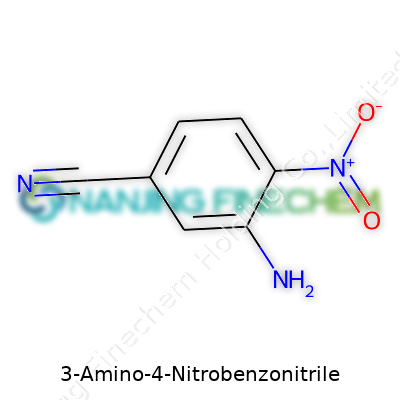
| Names | |
| Preferred IUPAC name | 3-amino-4-nitrobenzenecarbonitrile |
| Other names |
3-Amino-4-nitrobenzenecarbonitrile 4-Nitro-3-cyanoaniline 3-Amino-4-nitrobenzonitril 4-Nitro-m-cyanoaniline 3-Cyano-6-nitroaniline |
| Pronunciation | /ˈθriː-əˈmiːnoʊ-ˈfɔːr-ˈnaɪtroʊ-ˌbɛnzoʊˈnaɪtraɪl/ |
| Identifiers | |
| CAS Number | 63944-81-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **3-Amino-4-Nitrobenzonitrile**: ``` NC1=CC=C(C#N)C(C1)[N+](=O)[O-] ``` |
| Beilstein Reference | 109780 |
| ChEBI | CHEBI:73249 |
| ChEMBL | CHEMBL194491 |
| ChemSpider | 151364 |
| DrugBank | DB08275 |
| ECHA InfoCard | 100.044.279 |
| EC Number | 217-931-6 |
| Gmelin Reference | 142213 |
| KEGG | C14381 |
| MeSH | D064370 |
| PubChem CID | 68154 |
| RTECS number | BZ9800000 |
| UNII | C5P4N2C482 |
| UN number | Not assigned |
| CompTox Dashboard (EPA) | DTXSID40895677 |
| Properties | |
| Chemical formula | C7H5N3O2 |
| Molar mass | 163.13 g/mol |
| Appearance | Yellow solid |
| Odor | Odorless |
| Density | 1.36 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.04 |
| Vapor pressure | 8.14E-7 mmHg at 25°C |
| Acidity (pKa) | pKa = 1.62 |
| Basicity (pKb) | pKb = 9.06 |
| Magnetic susceptibility (χ) | -52.3·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.761 |
| Dipole moment | 4.72 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 178.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 235.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -641 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335, H410 |
| Precautionary statements | P261, P264, P270, P271, P272, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P363, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-2-🏠 |
| Flash point | 116°C |
| Lethal dose or concentration | Lethal dose or concentration: LD50 (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2000 mg/kg (rat, oral) |
| NIOSH | RN84953 |
| REL (Recommended) | 0.05 mg/m³ |
| Related compounds | |
| Related compounds |
4-Nitrobenzene-1,3-diamine 4-Nitrobenzonitrile 3-Nitro-4-aminobenzonitrile 3-Aminobenzonitrile 3-Amino-4-nitroaniline |